

 �ܴ���A��ķ�Ӧ��ϵ��T1�䵽T2��
�ܴ���A��ķ�Ӧ��ϵ��T1�䵽T2��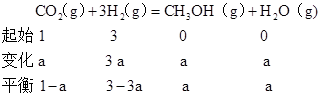
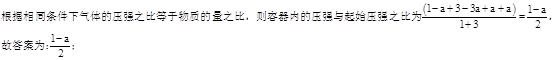
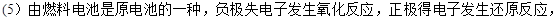
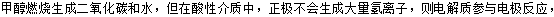
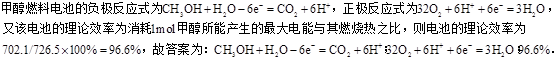


 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��X+Y=M+NΪ���ȷ�Ӧ������֪X��Y��������һ������M��N�������� |
| B��1molSO2�ļ����ܺʹ���1mol���1mol�����ļ���֮�� |
| C����C��ʯī��=C�����ʯ����H=+1.9KJ/mol��֪�����ʯ��ʯī�ȶ�] |
| D���������������������ֱ���ȫȼ�գ�ǰ�߷ų��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��3��д�����и�����ˮ������ӷ���ʽ��
��3��д�����и�����ˮ������ӷ���ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ��ѧ�� | N��H | N��N | O==O | N��N | O��H |
| ����(kJ/mol) | 386 | 167 | 498 | 946 | 460 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��KO2��ֻ�������Ӽ� |
| B���������صĻ�ѧʽΪKO2��ÿ����������1��K+��1��O2- |
| C����������ÿ��K+���������O2����6������ |
| D�������У�����ԭ��֮�䶼�������Ӽ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�� NH4Cl | B�� SiO2 |
| C�� P4 | D�� Na2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ��ѧ�� | H-H | N-H | N��N |
| ����/kJ��mol-1 | 436 | 391 | 945 |
 2NH3 ��H="a" kJ��mol-1���Ը��ݱ������м������ݹ���a����ֵΪ________��
2NH3 ��H="a" kJ��mol-1���Ը��ݱ������м������ݹ���a����ֵΪ________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����162 kJ��mol��1 | B����81 kJ��mol��1 |
| C����162 kJ��mol��1 | D����81 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com