
�����������壨FeSO4·7H2O����ҽҩ������Ѫ����ij����С��ⶨ�ò�Ѫ������Ԫ�صĺ�����������ò�Ѫ���Ƿ���ʡ�ʵ�鲽�����£�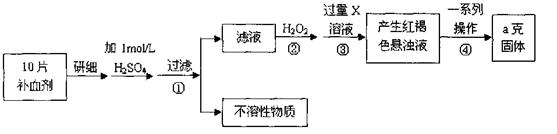
��ش��������⣺
��1���������Һ�еμ�KSCN��Һ����Һ��Ϊ��ɫ�������Һ�к��� (�����ӷ���)��������Һ�л�����Fe2+�ķ���Ϊ
��ע���Լ�������
��2������ڼ������H2O2��Ŀ���� ��
��3��������з�Ӧ�����ӷ���ʽΪ ��
��4���������һϵ�д����IJ���������������ˡ� �����ա� ��������
��5����ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص�����Ϊ g��
��1��Fe3+ ȡһ������Һ���μ�����KMnO4��Һ��KMnO4��Һ��ɫ
��2����Fe2+ȫ������ΪFe3+
��3��Fe3++3OH��= Fe��OH��3������Fe3++3NH3·H2O = Fe��OH��3+3NH4+��
��4��ϴ�� ��ȴ
��5��0.07a (��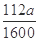 ���仯����ʽ����
���仯����ʽ����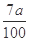 )
)
���������������1��KSCN��Һ��Fe3+��Ӧ��Ѫ��ɫ����Fe3+���ڵ�����������Fe2+�Ļ�ԭ�Լ�������ڣ�ȡһ������Һ���μ�����KMnO4��Һ��KMnO4��Һ��ɫ����2��H2O2�ǰ�Fe2+����ΪFe3+��������Ϊ�˽�Fe2+ȫ������ΪFe3+����3������Һ�м���ǿ����Һ��������������������4��Ϊ�˵õ�����������������Ҫϴ�ӣ����գ���ȴ��õ�����5������Ϊ��������10Ƭ��Ѫ���õ��ģ�ÿƬ��Ѫ��������Ԫ�ص�����Ϊa��160��56��2��10="0.07a" g��
���㣺���黯ѧʵ�鶨�������й����⡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������������Ҫ�������Σ���ũҵ������ũҩ����Ҫ��С����벡���������������ݼ����ڹ�ҵ������Ⱦɫ����������īˮ��ľ�ķ����ȡ�
��1�����Ƶ��̷���FeSO4��7H2O����dz��ɫ�ģ����ڿ����м��ױ�ɻ�ɫ������ɫ�ļ�ʽ������[Fe(OH)SO4]��д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2����֪FeSO4�ڲ�ͬ�����·ֽ�õ����ﲻͬ��������FeO��SO3��Ҳ������Fe2O3��SO3��SO2��
SO3�۵���16.8�棬�е���44.8�档
ij�о���ѧϰС����������װ�ý���ʵ��̽�����ڼ���������FeSO4�ķֽ�����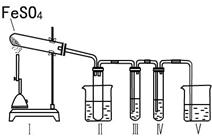
����װ�â�͢����������������Իش��������⣺
�٢�װ���ձ���ˮ���¶�Ӧ������ ��ѡ�0�桢25�桢50�桱����װ�â�������� ��
��װ�â��е��Լ������� ��ѡ����ţ���ͬ���������� ����֤����������к���SO2�� װ�â��е��Լ������� ��
| A��2 mol/LNa2CO3��Һ |
| B��Ʒ����Һ |
| C��0.5 mol/LBaCl2��Һ |
| D��0.5 mol/LBa(NO3)2 |
| �������� | Ԥ��ʵ������ | Ԥ��ʵ����� |
| ������һ����Һ�м��� �� | | �����к���Fe2O3 |
| ����һ����Һ�еμ�2�λ�ɫK3[Fe(CN)6]��Һ�� | ������ɫ���� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
С����ϲ����ѧʵ��Σ�����Ҫѧϰ��̽�������仯����������Ի�ԭ�ԡ���������һ���߽����Ļ�ѧ���á�
��1����ǰ��ʦ����������Ԥϰ��ҵ������һ����ɣ�
������ͬ��̬�����ʸ�дһ��(�������Ԫ�صĻ��ϼ�)��_____��_______�� ________��
��д��һ������֮���ת��(�����ּ�̬)�Ļ�ѧ����ʽ��___________________ ��
��2��ʵ�����ṩ�������Լ���п�������ۡ�0.1 mol��L��1 FeCl3��Һ��0.1 mol��L��1 FeCl2��Һ��KSCN��Һ��������ˮ��̽��Fe2����Fe3���������ԡ���ԭ�ԡ�
����������ԭ��Ӧ���й�ԭ����С��˵Fe2�����л�ԭ�����������ԣ�Ϊ֤ʵ�Լ��ļ��裬�����С��һ�����ʵ�鷽��������ʵ�鲢����ʵ������������б���
| ̽������ | ʵ�鷽�� | ʵ������ |
| ̽��Fe2�����л�ԭ�� | ȡ����0.1 mol��L��1 FeCl2��Һ����������__________��������Һ�м�������__________ | ��Һ���Ѫ��ɫ |
| ̽��Fe2������������ | ȡ����0.1 mol��L��1 FeCl2�� Һ������_________��� ��Ӧ | ��Һ��dz��ɫ����ɫ ��������Ӧ���ӷ���ʽΪ________________ [��Դ:Z|xx|k.Com] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�̷���FeSO4��7H2O��������ȱ����ƶѪҩƷ����Ҫ�ɷ֡���������������м�����������������������ʣ�Ϊԭ�����������̷���һ�ַ�����
��֪�������±���H2S��Һ��pHԼΪ3.9��SnS������ȫʱ��Һ��pHΪ1.6��FeS��ʼ����ʱ��Һ��pHΪ3.0��������ȫʱ��pHΪ5.5��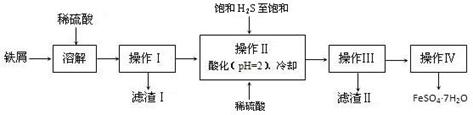
��1�������Ƶõ��̷��������Ƿ���Fe3+��ʵ������� ��
��2������II�У�ͨ�����������͵�Ŀ���� ������Һ���������ữ��pH=2��Ŀ���� ��
��3������IV��˳������Ϊ�� ����ȴ�ᾧ�� ��
��4���ⶨ�̷���Ʒ��Fe2+ �����ķ����ǣ�
a����ȡ2.8500g�̷���Ʒ���ܽ⣬��250mL����ƿ�ж��ݣ�
b����ȡ25.00mL������Һ����ƿ�У�
c���������ữ��0.01000mol?L-1KMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00mL��
����֪KMnO4������Һ��Fe2+ ��Ӧʱ����ԭΪMn2+����д���÷�Ӧ�����ӷ���ʽ�� ��
�ڼ���������Ʒ��FeSO4?7H2O����������Ϊ ����С����ʾ��������λС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
FeCl3���ִ���ҵ������Ӧ�ù㷺��ij��ѧ�о���ѧϰС��ģ�ҵ�����Ʊ���ˮFeCl3�����ø���ƷFeCl3��Һ�����ж���H2S��
I�����������ϵ�֪����ˮFeCl3�ڿ������׳��⣬����������������������Ʊ���ˮ FeCl3��ʵ�鷽����װ��ʾ��ͼ�����ȼ��г�װ����ȥ���������������£�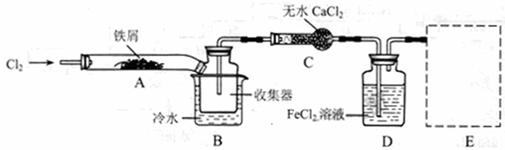
�ټ���װ�õ������ԣ�
��ͨ������Cl2���Ͼ�װ���еĿ�����
���þƾ�������м�·���������Ӧ��ɣ�
�ܡ���
����ϵ��ȴ��ֹͣͨ��Cl2�����ø����H2�Ͼ�Cl2�����ռ����ܷ⡣
��ش��������⣺
��1��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ ��
��2���ڢ۲����Ⱥ����ɵ���״FeCl3�ֽ����ռ��������������ڷ�Ӧ��A�Ҷˡ�Ҫʹ������FeCl3�����ռ������ڢܲ������� ��
��3�����������У�Ϊ��ֹFeCl3��������ȡ�Ĵ�ʩ�У������ţ� ��
��4�����û��װ��C����ƣ��ᵼ�� ��
��5�������ӷ���ʽ��ʾ���߿�E��������װ�ú��Լ������ã� ��
��6����װ��D�еĸ���ƷFeCl3��Һ����H2S���õ�������
��д����Ӧ�����ӷ���ʽ�� ��
��Ӧ�������ռ��������ù�����ȫ����ϡ���ᣬС��ͬѧ��������Һ���������ӵijɷ������ֹ۵㣺��ֻ��Fe3+����ֻ��Fe2+���� ��
Ϊ̽����Һ����ɣ�ʵ�����£�
| ʵ�鲽�� | ʵ������ | ʵ����ۼ���Ӧ���ӷ���ʽ |
| ��ȡ����������Һ���Թ��У���������KSCN��Һ�� | | ˵��������ڲ�����������ٻ�۳����������ӷ���ʽ�� �� |
| ����ȡ����������Һ���Թ��У������������� KMnO4��Һ�� | ��Һ�Ϻ�ɫ��ȥ | ˵���� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��֪���ӷ�Ӧ��Fe3����3SCN�� Fe��SCN��3���з�ӦѸ�١��������Ե��ص㣬�Ǽ���Fe3�����õķ���֮һ��ij��ѧ��ȤС��Ϊ̽��Fe��SCN��3�����ʣ���������ʵ�飺
Fe��SCN��3���з�ӦѸ�١��������Ե��ص㣬�Ǽ���Fe3�����õķ���֮һ��ij��ѧ��ȤС��Ϊ̽��Fe��SCN��3�����ʣ���������ʵ�飺
��ȡ10 mL l mol��L��1FeCl3��Һ���μ�3��4��ŨKSCN��Һ������Һ������ɺ�
ɫ��
��ȡ������ɫ��Һ���μ�����Ũ���ᣬ���ã���Һ��ɫ��ȥ��ͬʱ���������ĺ���ɫ��
������A��
�۽�����������Aͨ�������Ba��OH��2��Һ�У�������ɫ����B��ʣ������C��
����C��ɫ��ζ����ʹȼ�յ�ľ��Ϩ�𣬿��ŷŵ������У�����ı�����ijɷ֡�
�ܹ��ˣ����ɫ����B�еμ�����ϡ���ᣬ������ȫ�ܽ⣬ͬʱ������ʹ����ʯ��ˮ��
���ǵ���ɫ��ζ����D��
��ȡ���з�Ӧ����Һ�������μ�BaCl2��Һ������������ϡ����İ�ɫ����E��
��������ʵ�����ش��������⣺
��1��B�Ļ�ѧʽΪ_________________��E�Ļ�ѧʽΪ___________________��
��2���������A�ijɷ���___________________���ѧʽ����
��3������ȤС��ͬѧ��������ʵ����������ó����ۣ�Fe��SCN��3���л�ԭ�ԣ���ʵ����з�Ӧʱ��������Ԫ����____________����Ԫ�ط��ţ�������Ԫ��C��S��N��ԭ�ӽṹ���ۼ������֪ʶ�ƶϳ�SCN���ĽṹʽΪ_________________��
��4��ʵ����з�Ӧ�����ӷ���ʽΪ___________________________________________��
��5������ȤС��ͬѧ������ʵ���еõ�����������SCN����Ӽ���Fe2��ʱӦע��_________________________________________________________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����仯���������������������Ź㷺��Ӧ�á�
��1������������Ҫ��ҵ���ϣ��÷���м�Ʊ������������£��ش��������⣺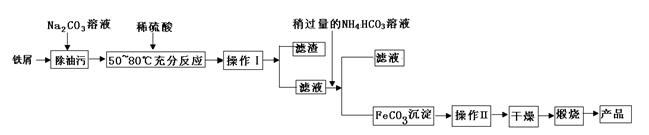
�ٲ���������Ʒֱ���____��____��
��д���ڿ���������FeCO3�Ļ�ѧ����ʽ ��
�������������֣���Ʒ�н���Fe2+���ڣ������ʵ������Ʒ������Fe2+��
��2����Щͬѧ��ΪKMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ����ȡ2.850g�̷���FeSO4��7H2O����Ʒ���ܽ⣬��250mL����ƿ�ж��ݣ���ȡ25.00mL������Һ����ƿ�У��������ữ��0.01000mol/LKMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00mL��
��д������KMnO4��Һ��FeSO4��Һ��Ӧ�����ӷ���ʽ
�ڼ���������Ʒ��FeSO4��7H2O����������Ϊ [��֪M(FeSO4��7H2O)=278g/mol]
�۵ζ��ﵽ�յ�ʱ��ƿ����Һ��ɫ�仯Ϊ
�����в����ᵼ����Ʒ��FeSO4��7H2O�����������IJⶨ���ƫ�ߵ���_____________��
a��δ������ƿ
b��ʢװ��Һ�ĵζ���û���ñ�Һ��ϴ
c�� �ζ��յ�ʱ�ζ��ܼ����в�������
d����ȡ����Һ������ע�ӿ̶�ʱ��ʼƽ�ӡ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ͼ����ע�����м�������Na2SO3���壬����������Ũ����(�Բ��Ӵ�ֽ��Ϊ)���������й�˵����ȷ����(����) 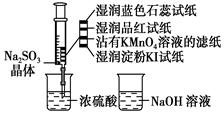
| A����ɫʯ����ֽ�ȱ�����ɫ |
| B��Ʒ����ֽ��մ��KMnO4��Һ��ֽ����ɫ����֤��SO2��Ư���� |
| C��ʪ�����KI��ֽδ����˵��SO2������������I2 |
| D��NaOH��Һ��Ʒ����Һ�������ڳ�ȥʵ���ж����SO2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com