
| �� �� �� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | �� | �� | ||||||
| �� | �� | �� | �� | �� | �� | �� | ||
| �� | �� | �� |
 ��
������ ��Ԫ�������ڱ��е�λ�ÿ�֪����ΪN����ΪF����ΪNa����ΪMg����ΪAl����ΪSi����ΪCl����ΪAr����ΪK����ΪBr��
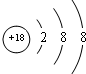
��1��ϡ������Ar�Ļ�ѧ��������ã�ԭ�Ӻ��������Ϊ18�����ݺ�������Ų�����ԭ�ӽṹʾ��ͼ��
��2���ؿ��к������Ľ���Ԫ��������
��3��������γɻ�����ΪMgF2����þ����������ӹ��ɣ�
��4��FԪ��û����ۺ����ᣬ����������Ӧ��ˮ�����У�������ǿ���Ǹ����ᣬK�Ľ�������ǿ��KOH�ļ�����ǿ��
��5�������������Ʒ�Ӧ����ƫ�������������������������������Ʒ�Ӧ����ƫ��������ˮ��
��6���ں͢��γɵĻ�����ΪSiF4���ں͢��γɵĻ�����ΪKF��
��� �⣺��Ԫ�������ڱ��е�λ�ÿ�֪����ΪN����ΪF����ΪNa����ΪMg����ΪAl����ΪSi����ΪCl����ΪAr����ΪK����ΪBr��
��1��ϡ������Ar�Ļ�ѧ��������ã�ԭ�Ӻ��������Ϊ18��ԭ�ӽṹʾ��ͼΪ�� ��
��
�ʴ�Ϊ�� ��
��
��2���ؿ��к������Ľ���Ԫ���������ʴ�Ϊ������
��3��������γɻ�����ΪMgF2����þ����������ӹ��ɣ��õ���ʽ��ʾ�γɹ���Ϊ�� ��
��
�ʴ�Ϊ�� ��
��
��4��FԪ��û����ۺ����ᣬ����������Ӧ��ˮ�����У�������ǿ����HClO4��K�Ľ�������ǿ��KOH�ļ�����ǿ��
�ʴ�Ϊ��HClO4��KOH��
��5�������������Ʒ�Ӧ����ƫ����������������Ӧ����ʽΪ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
�����������������Ʒ�Ӧ����ƫ��������ˮ����Ӧ���ӷ���ʽΪ��Al ��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����Al ��OH��3+OH-=AlO2-+2H2O��
��6���ں͢��γɵĻ�����ΪSiF4���ں͢��γɵĻ�����ΪKF��ǰ�ߺ��й��ۼ������ߺ������Ӽ���
�ʴ�Ϊ�����ۼ������Ӽ���
���� ���⿼��Ԫ�����ڱ���Ԫ���������ۺ����ã���������Ԫ�����ڱ��Ľṹ�������ڻ���֪ʶ�Ĺ��̣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | RO | B�� | R2Om | C�� | R2Om-n | D�� | R2O2m-n |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
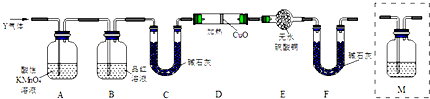
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
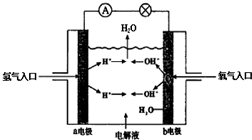
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ǿ����HIO4��HBrO4��HClO4 | B�� | ԭ�Ӱ뾶��С��Al��P��N | ||
| C�� | ����ǿ����KOH��NaOH��LiOH | D�� | ������ǿ����Na��Mg��Al |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
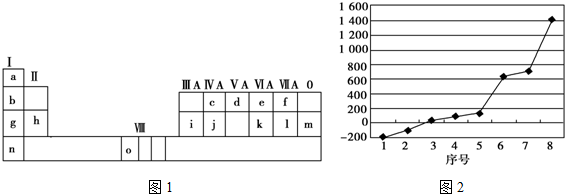
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\frac{12n}{m}$ | B�� | $\frac{60n}{m}$ | C�� | $\frac{11m}{120n}$ | D�� | $\frac{120n}{11m}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| Ԫ�ش��� | A | B | C | D | E |
| ԭ�Ӱ뾶/nm | 0.160 | 0.143 | 0.102 | 0.071 | 0.099 |
| ��Ҫ���ϼ� | +2 | +3 | +6��-2 | -1 | -1 |
| A�� | A��B�����Ӱ뾶��С��ϵΪ��B��A | |
| B�� | D��E�γɵļ����ӵĻ�ԭ�ԣ�E��D | |
| C�� | ��̬�⻯����ȶ��ԣ�D��C | |
| D�� | ����������Ӧ��ˮ��������ԣ�C��E |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ɫǿ������Һ�п��ܴ������� Al3+��NH4+��Cl?��S2? | |
| B�� | ������Һ�п��ܴ������� Na+��ClO?��SO42?��I? | |
| C�� | ��������Һ�п��ܴ������� Na+��K+��Cl?��CO32? | |
| D�� | ������Һ�п��ܴ������� Ba2+��K+��Cl?��SO42? |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com