
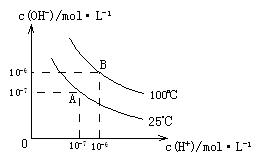
��ˮ�ĵ���ƽ���У�![]() ��
��![]() �Ĺ�ϵ����ͼ��ʾ��
�Ĺ�ϵ����ͼ��ʾ��
��1��A��ˮ�����ӻ�Ϊ1��10-14��B��ˮ�����ӻ�Ϊ �����ˮ�����ӻ��仯��ԭ���� ��
��2��25��ʱ��![]() ��ˮ��Һ�еĵ��뷽��ʽΪ��
��ˮ��Һ�еĵ��뷽��ʽΪ��
![]() ��
��![]()
![]() ��
��
�� 0.1 mol/L![]() ��Һ��pH 1�����������������������
��Һ��pH 1�����������������������
����0.1 mol/L![]() ��Һ�У�������Ũ���ɴ�С��˳���ǣ�
��Һ�У�������Ũ���ɴ�С��˳���ǣ�
��
��3��100��ʱ��0.01 mol/L![]() ��Һ��pH = ��
��Һ��pH = ��
��4��100��ʱ����pH =8��![]() ��Һ��pH =5��ϡ�����ϣ�������100����£���ʹ�����Һ��pH =7����
��Һ��pH =5��ϡ�����ϣ�������100����£���ʹ�����Һ��pH =7����![]() ��Һ������������Ϊ ��
��Һ������������Ϊ ��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ�ĵ���ƽ���У�![]() ��
��![]() �Ĺ�ϵ����ͼ��ʾ��
�Ĺ�ϵ����ͼ��ʾ��
��1��A��ˮ�����ӻ�Ϊ1��10-14��B��ˮ�����ӻ�Ϊ �����ˮ�����ӻ��仯��ԭ���� ��
��2��25��ʱ��![]() ��ˮ��Һ�еĵ��뷽��ʽΪ��
��ˮ��Һ�еĵ��뷽��ʽΪ��
![]() ��
��![]()
![]()
![]() ��
��
�� 0.1 mol/L![]() ��Һ��pH 1�����������������������
��Һ��pH 1�����������������������
����0.1 mol/L![]() ��Һ�У�������Ũ���ɴ�С��˳���ǣ�
��Һ�У�������Ũ���ɴ�С��˳���ǣ�
��
��3��100��ʱ��0.01 mol/L![]() ��Һ��pH = ��
��Һ��pH = ��
��4��100��ʱ����pH =8��![]() ��Һ��pH =5��ϡ�����ϣ�������100����£���ʹ�����Һ��pH =7����
��Һ��pH =5��ϡ�����ϣ�������100����£���ʹ�����Һ��pH =7����![]() ��Һ������������Ϊ ��
��Һ������������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꺣��ʡ�λ���ѧ�߶���ѧ�ڵڶ����¿���ѧ�Ծ� ���ͣ������
��ˮ�ĵ���ƽ���У� ��
�� �Ĺ�ϵ����ͼ��ʾ��
�Ĺ�ϵ����ͼ��ʾ��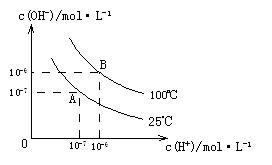
��1��A��ˮ�����ӻ�Ϊ1��10-14��B��ˮ�����ӻ�Ϊ �����ˮ�����ӻ��仯��ԭ���� ��
��2��25��ʱ��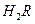 ��ˮ��Һ�еĵ��뷽��ʽΪ��
��ˮ��Һ�еĵ��뷽��ʽΪ��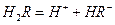 ��
��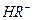

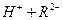 ��
��
�� 0.1 mol/L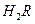 ��Һ��pH 1�����������������������
��Һ��pH 1�����������������������
����0.1 mol/L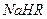 ��Һ�У�������Ũ���ɴ�С��˳���ǣ�
��Һ�У�������Ũ���ɴ�С��˳���ǣ�
��
��3��100��ʱ��0.01 mol/L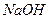 ��Һ��pH = ��
��Һ��pH = ��
��4��100��ʱ����pH =8��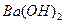 ��Һ��pH =5��ϡ�����ϣ�������100����£���ʹ�����Һ��pH =7����
��Һ��pH =5��ϡ�����ϣ�������100����£���ʹ�����Һ��pH =7����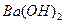 ��Һ������������Ϊ ��
��Һ������������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꺣��ʡ�߶���ѧ�ڵڶ����¿���ѧ�Ծ� ���ͣ������
��ˮ�ĵ���ƽ���У�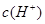 ��
��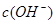 �Ĺ�ϵ����ͼ��ʾ��
�Ĺ�ϵ����ͼ��ʾ��
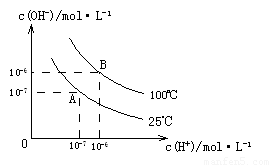
��1��A��ˮ�����ӻ�Ϊ1��10-14��B��ˮ�����ӻ�Ϊ �����ˮ�����ӻ��仯��ԭ���� ��
��2��25��ʱ��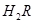 ��ˮ��Һ�еĵ��뷽��ʽΪ��
��ˮ��Һ�еĵ��뷽��ʽΪ��
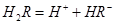 ��
��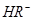
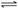
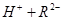 ��
��
�� 0.1 mol/L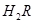 ��Һ��pH
1�����������������������
��Һ��pH
1�����������������������
����0.1 mol/L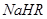 ��Һ�У�������Ũ���ɴ�С��˳���ǣ�
��Һ�У�������Ũ���ɴ�С��˳���ǣ�
��
��3��100��ʱ��0.01 mol/L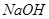 ��Һ��pH = ��
��Һ��pH = ��
��4��100��ʱ����pH =8��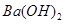 ��Һ��pH =5��ϡ�����ϣ�������100����£���ʹ�����Һ��pH =7����
��Һ��pH =5��ϡ�����ϣ�������100����£���ʹ�����Һ��pH =7����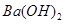 ��Һ������������Ϊ ��
��Һ������������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��ĩ�� ���ͣ������
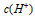 ��
��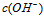 �Ĺ�ϵ����ͼ��ʾ
�Ĺ�ϵ����ͼ��ʾ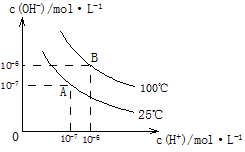
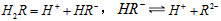 ��
�� ��Һ�У�������Ũ���ɴ�С��˳���ǣ�_____________________��
��Һ�У�������Ũ���ɴ�С��˳���ǣ�_____________________��  ��Һ��pH =_________________��
��Һ��pH =_________________��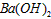 ��Һ��pH =5��ϡ�����ϣ�������100����£���ʹ�����Һ��pH=
��Һ��pH =5��ϡ�����ϣ�������100����£���ʹ�����Һ��pH=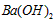 ��Һ������������Ϊ____________________��
��Һ������������Ϊ____________________�� �鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com