
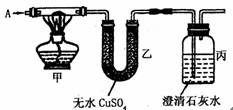
����ͼ��ʾ����֪A�dz����Ĵ������壬���к�ɫ��ĩΪ����ͭ��̿�ۻ����ߵĻ���
�Իش��������⣺
��1����AΪCO2���ڱ��е�����ȼ�����壬����з�����Ӧ�ķ�Ӧ����ʽ
��2�������к�ɫ��ĩΪ����ͭ��ʵ������м״���ɫ��ĩ��ɺ�ɫ���Ҵ���ˮCuSO4�ޱ仯����ͨ���AΪ �����г��ֵ�����Ϊ �����з�����Ӧ�����ӷ���ʽΪ
��3����������ˮCuSO4�����ɫ�����г��ְ�ɫ���ǣ���ͨ���AΪ ����ɫ��ĩ�ijɷ���

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������ʮ����2009��2010ѧ��ȵ�һѧ����ĩ�Ծ�������ѧ ���ͣ�022
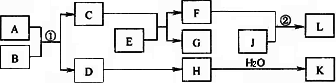
����A��K��ת����ϵ����ͼ��ʾ����֪A��������Ԫ����ɵĻ����B�Ƿǽ������ʣ�J�ǽ������ʣ�H����Է���������D��16(���ַ�Ӧ����������г�)��
����������Ϣ���ش��������⣺
����AΪ���壬F���Σ���Ӧ������ҵ����ȡK����Ҫ��Ӧ����Ӧ��Ϊ���Ϸ�Ӧ��
(1)G�ĵ���ʽ��_____________________��
(2)��ҵ�Ϸ�Ӧ����________(�ҵ�豸����)�н��У�
(3)��Ӧ�ٵĻ�ѧ����ʽΪ____________��
����AΪ���壬EΪ���ý������ʣ���K�롰I���е�K��ͬ��
(4)����A�ķ���ʽ��________________��
(5)��Ӧ�ڵ����ӷ���ʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������Ϣ���ش��������⣺
��.��AΪ���壬F���Σ���Ӧ���ǹ�ҵ����ȡK����Ҫ��Ӧ����Ӧ��Ϊ���Ϸ�Ӧ��
��1��G�ĵ���ʽ��_______________��
��2����ҵ�Ϸ�Ӧ����_______________���ҵ�豸���ƣ��н��С�
��3����Ӧ�ٵĻ�ѧ����ʽΪ_____________________________________________��
��.��AΪ���壬EΪ���ý������ʣ���K�롰���е�K��ͬ��
��4������A�ķ���ʽ��_______________��
��5����Ӧ�ڵ����ӷ���ʽΪ_____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����A��K��ת����ϵ����ͼ��ʾ����֪A��������Ԫ����ɵĻ����B�Ƿǽ������ʣ�J�ǽ������ʡ�H����Է���������D��16�����ַ�Ӧ�������δ�г�����

����������Ϣ���ش��������⣺
��.��AΪ���壬F���Σ���Ӧ���ǹ�ҵ����ȡK����Ҫ��Ӧ����Ӧ��Ϊ���Ϸ�Ӧ��
�� G�ĵ���ʽ��__________��
�� ��ҵ�Ϸ�Ӧ���� ���ҵ�豸���ƣ��н��С�
�� ��Ӧ�ٵĻ�ѧ����ʽΪ ��
��. ��AΪ���壬EΪ���ý������ʣ���K�롰���е�K��ͬ��
�� ����A�ķ���ʽ��____________��
�� ��Ӧ�ڵ����ӷ���ʽΪ____________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com