
��һ��������þ���Ͻ�Ͷ��100 mLһ��Ũ�ȵ������У��Ͻ���ȫ�ܽ⡣��������Һ�еμ�Ũ��Ϊ5 mol/L��NaOH��Һ�����ɵij����������NaOH��Һ�������ϵ��ͼ���������������λ��mL��������������λ��g����
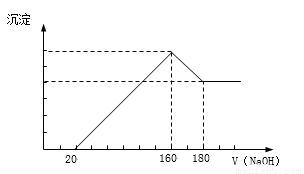
��1������NaOH��Һ0--20mL�����з�Ӧ����ʽΪ��_______________��160--180mL�����з�Ӧ����ʽΪ___________________��
��2���Ͻ���Mg������Ϊ____________g������HCl�����ʵ���Ũ��Ϊ____________mol/L��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ������ѧ��9.18�ܿ���ѧ�Ծ��������棩 ���ͣ������
�����仯�������������������м�����Ҫ�����á�
��1���ҹ�����ϵ�����ػ������(N2H4)��ȼ�ϡ�N2H4��NH3�����ƵĻ�ѧ���ʡ�
��д���������ᷴӦ�����ӷ���ʽ��_______________��
���ڻ���ƽ�����װ��Һ̬�º�˫��ˮ�������ǻ��ʱѸ�ٷ�Ӧ���ɵ�����ˮ������д����Ӧ�Ļ�ѧ����ʽ��______________��
�ۻ������ʱ����Ϊȼ�ϣ�Ҳ������һ�����������������ڴ˷�Ӧ��������ת��2 mol���ӣ�������ȼ���µ�����Ϊ__________��
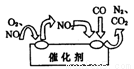
��2������β���еĵ����������γ����ꡢ�������ж�����֮һ��Ϊ�˼�����Ⱦ���ɳ���ʹ������β������װ�ã���ԭ����ͼ��ʾ��д�������������ܷ�Ӧ�Ļ�ѧ����ʽ��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ������ѧ��һ�����Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ͼ����ˮ��Ӧ��ʵ���У�������������ȷ���ǣ� ��

A���Թ��е��������Ƴ��ŵ�ϸ��˿����
B�����Թܵײ���ʪ���Ƶ��Թܿڲ�
C�������۸�����ʯ�����ϣ���������ˮ�����ĽӴ����
D����ȼ��������֤����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʦ���и�����ѧ����ѧ�����Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���ᱵ1(CH3COO)2Ba��H2O]��һ��ýȾ�����������й�0.1 mol/L���ᱵ��Һ������Ũ�ȵıȽϣ����в���ȷ����
A��c(Ba2��)��c(CH3COO��)��c(OH��)��c(H��)
B��c(H��)��2c(Ba2��)��c(CH3COO��)��c(OH��)
C��c(H��)��c(OH��)��c(CH3COOH)
D��2c(Ba2��)��c(CH3COO��)��c(CH3COOH)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʦ���и�����ѧ����ѧ�����Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
ijKCl��Ʒ�к�������K2CO3��K2SO4�Ͳ�����ˮ�����ʡ�Ϊ���ᴿKCl���Ƚ���Ʒ��������ˮ�У����衢���ˣ��ٽ���Һ����ͼ��ʾ��������ᴿ(���˲�������ȥ)������˵����ȷ����
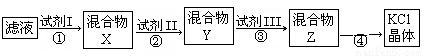
A����ʼ��Һ������pH��7 B���Լ���ΪBa(NO3)2��Һ
C����ͼ�����뾭2�ι��� D�������Ŀ���dz�ȥCO32��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и�����ѧ��9���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����ͭ��������ӻ�ͭ��CuFeS2����ʼ����֪��ͭ������Ϊ+2�ۡ���ͭ��ı��չ����з�������Ҫ��ӦΪ��2CuFeS2+O2=Cu2S+2FeS+SO2������˵������ȷ����
A��SO2���������������ǻ�ԭ����
B��CuFeS2�������������ǻ�ԭ��
C��O2ֻ��������
D������1mol O2�μӷ�Ӧ����Ӧ����4mol����ת��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и�����ѧ��9���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪��������þ������Ũ�Ⱦ�Ϊ3mol/L�Ģ������NH4Cl��Һ�۴������Һ�����ԣ�������˵����ȷ���ǣ� ��
A��c(NH4+)���� �� ��
B��pH���� �� �� �� ��
C��Mg(OH)2���ڢڵ���Ҫԭ��������NH4+ˮ��ʹ��Һ�����ԣ�����ƽ��Mg(OH)2(s)  Mg2+ (aq) + 2OH- (aq)���ܽⷽ���ƶ�
Mg2+ (aq) + 2OH- (aq)���ܽⷽ���ƶ�
D��������ٺ͢ۻ����Һ�� c(Cl��) ��c(NH4+) �� c(H+) �� c(CH3COO��) �� c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и�����ѧ��9���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���е��ʻ������ʵ�������ȷ����
A��ʳƷ��װ�г������й轺�����۵�С������ֹʳ���ܳ�����������
B��Na2O��Na2O2���Ԫ����ͬ����CO2��Ӧ����Ҳ��ͬ
C��SiO2���ᡢ����ܷ�Ӧ����������������
D��������ˮ�����ԣ������еμ�������ɫʯ����Һ���������Һ�ʺ�ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�������տ�������һ����ѧ�����ϵ�һ���¿���ѧ���������棩 ���ͣ�ѡ����
��֪������KClO3�����еμ�Ũ���ᣬ��������ɫ���壻����NaI��Һ��ͨ������ʵ��ٲ��������壬��Һ���ɫ����ȡʵ������ɵ���Һ���ڵ���KI��ֽ�ϣ���ֽ����ɫ�������жϲ���ȷ����( )
A��ʵ���˵��KI������
B��ʵ������������뻹ԭ�������ʵ���֮��Ϊ1��2
C��ʵ���֤��Cl�����л�ԭ��
D������ʵ֤�������ԣ�ClO3����Cl2��I2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com