
 ����(N2H4) Ϊȼ�ϡ���������Ϊ��ȼ������֪���и����ʷ�Ӧ���Ȼ�ѧ����ʽ��
����(N2H4) Ϊȼ�ϡ���������Ϊ��ȼ������֪���и����ʷ�Ӧ���Ȼ�ѧ����ʽ��| A��N2H4(g)+2H2O2(l)= N2(g)+4H2O(l)��H����817.63 kJ��mol��1 |
| B��N2H4(g)+2H2O2(l)= N2(g)+4H2O(g)��H����817.63 kJ��mol��1 |
| C��N2H4(g)+2H2O2(l)= N2(g)+4H2O(l)��H����641.63 kJ��mol��1 |
D��N2H4(g)+2H2O2(l)= N2(g)+4H2O( g)��H����641.63 kJ��mol��1 g)��H����641.63 kJ��mol��1 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ˮʱ����22.68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ________________ ________________��
ˮʱ����22.68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ________________ ________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����10 NA������ת��ʱ���÷�Ӧ�ų�1300kJ������ |
| B����1 NA��ˮ����������ΪҺ��ʱ������1300kJ������ |
| C����2 NA��̼�����õ��Ӷ�����ʱ���ų�1300kJ������ |
| D����8 NA��̼�����õ��Ӷ�����ʱ���ų�1300kJ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��2H2(g)+ O 2(g) =" " 2H2O(1) H =" " ��142.9kJ��mol��l H =" " ��142.9kJ��mol��l |
B��2H2(g)+ O 2(g) =" " 2H2O(1) H =" " ��571.6kJ��mol��l H =" " ��571.6kJ��mol��l |
C��2H2+O2 = 2H2O H = ��571.6lkJ��mol��l H = ��571.6lkJ��mol��l |
D��H2(g)+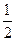 O 2(g) = H2O(1) O 2(g) = H2O(1) H = +285.8kJ��mol��l H = +285.8kJ��mol��l |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 4NO(g)��6H2O(g)�� ��H����905 kJ/mol������
4NO(g)��6H2O(g)�� ��H����905 kJ/mol������ 2N2(g)��6H2O(g)����H ����1268 kJ/mol�����ڣ�
2N2(g)��6H2O(g)����H ����1268 kJ/mol�����ڣ�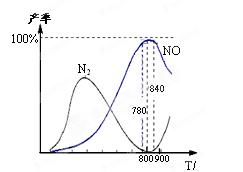
| A����ҵ�Ͻ��а����������� NOʱ���¶�Ӧ������780��840��֮�� |
| B����ҵ�ϲ������ϱ�n(O2)��n(NH3)��1.7��2.0����Ҫ��Ϊ����߷�Ӧ���� |
| C���ڼ�ѹ�������������������5��6��������Ϊ��ѹ�����ת���� |
D����������ΪNO���Ȼ�ѧ����ʽΪ��N2(g)��O2(g) 2NO(g) 2NO(g) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
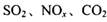 �ǶԻ���Ӱ��ϴ�Ķ������壬�����ǵĺ������ƺ��������Ż��������滷������Ч;����
�ǶԻ���Ӱ��ϴ�Ķ������壬�����ǵĺ������ƺ��������Ż��������滷������Ч;���� Ũ�ȵ���__________ (ѡ����ĸ)��
Ũ�ȵ���__________ (ѡ����ĸ)��


 �����£�
�����£�
 ,��ƽ�ⳣ��K=13.3��
,��ƽ�ⳣ��K=13.3�� ����
���� =_______________(������λ��Ч����)��
=_______________(������λ��Ч����)��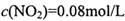 ��
�� ����ı��������____________________
����ı��������____________________�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B���ڢ� | C���ڢ� | D���٢� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com