
�����10�֣�
2005���ŵ������ѧ���������ϩ�����ֽⷴӦ�о���������ͻ������3λ��ѧ�ҡ�ϩ�����ֽⷴӦʵ������һ��������ϩ����̼̼˫�������ŵĻ�λ��
�磺2CH2=CHCH2CH3 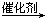 CH2��CH2+CH3CH2CH=CHCH2CH3��
CH2��CH2+CH3CH2CH=CHCH2CH3��
����֪������ȩ������һ�������¿����ȷ����ӳɷ�Ӧ��������ȥ��Ӧ��
�ֽ��Ա�ϩΪ�л�ԭ�ϣ��������з�Ӧ���Էֱ�ϳ���Ҫ�Ļ���ԭ��F��K����F��KΪԭ�Ͽɺϳ�һ����״�߷��ӻ�����M���仯ѧ���Ϊ(C12H20O4)n��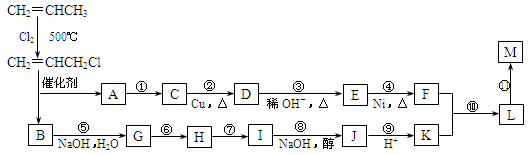
�ش��������⣺
43����Ӧ�ٵķ�Ӧ������_______________��
44����Ӧ�ޡ�������һ��Ӧ����HCl�ӳɣ��÷�Ӧ��_____���Ӧ��ţ��������һ����Ӧ��Ŀ����_____________________________________________________��
45������M�Ľṹ��ʽΪ��______________________________________��
46��д�����з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ�ࣺ_____________________________________________________________��
��Ӧ�⣺_____________________________________________________________��
43. �ӳɷ�Ӧ ��1�֣�
44. �ޣ�1�֣� ����B(��G)�����е�C��C����������2�֣�
45. ��2�֣�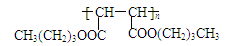
46. HOOCCH2CHClCOOH+3NaOH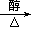 NaOOCCH��CHCOONa+NaCl+3H2O ��2�֣�
NaOOCCH��CHCOONa+NaCl+3H2O ��2�֣�
2CH3(CH2)3OH+HOOCCH��CHCOOH CH3(CH2)3OOCCH��CHCOO(CH2)3CH3+2H2O ��2�֣�
CH3(CH2)3OOCCH��CHCOO(CH2)3CH3+2H2O ��2�֣�
����������������������жϵڶ�����Ӧ������Ϣ�ṩ�Ļ��Ž�����Ӧ������Ϊ��ϩ��ClCH2CH=CHCH2Cl��
���㣺����STS�л��������й����⡣B�ܷ���ˮ�ⷴӦΪ���ߣ�AΪ��ϩ��CΪ��ϩ��ˮ�����Ҵ���DΪ��ȩ��D��E������Ϣ�ṩ��ȩ�ķ�Ӧ����CH3CH=CHCHO��FΪCH3CH2CH2CH2OH��Bˮ��ΪHOCH2CH=CHCH2OH��ͨ���ӳ�HCl����̼̼˫�����������ǻ�Ϊ�Ȼ�����ȥ�γ�̼̼˫������F������Ӧ�õ�L����Ӿ۵õ�M��
���㣺�����л���ѧ�ƶ��й����⡣


 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
ͼ�и���������ѧ��ѧ�г��������ʣ��ס��Ҿ������ӻ���������������Ӹ�����Ϊ1��1�����Ƿ��ͷ۵���Ҫ�ɷ֣�����һ�ֳ��õĻ��ʣ�B��D���³�ѹ�������塣��ش��������⣺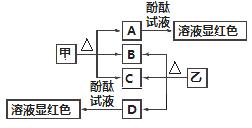
��1����������________��
��2��A��D���ʵ�ˮ��Һ�ֱ�����̪��Һ����Һ���Ժ�ɫ����ԭ��________(���ͬ������ͬ��)��
��3�����ڳ�ʪ�Ŀ����лỺ���ֽ⣬A�����տ����е�ˮ�֣�A��nH2O===A��nH2O(nΪƽ��ֵ��n��10)��ȡû�����Ʊ��ܵļ���Ʒ9.16 g������ˮ�Ƴ���Һ����������ϡ���Ტ��ͣ�ؽ��裬�����������������ɵ�B�����(��״��)���±���(����ˮ�е�B����)
| ��������(mL) | 4 | 8 | 15 | 20 | 50 | 120 | 150 |
| ����B�����(mL) | 0 | 0 | 112 | 224 | 896 | 2240 | 2240 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
����ѧ����ѡ��5���л���ѧ������(15��)
������H��������·�ߺϳɣ�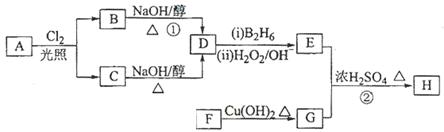
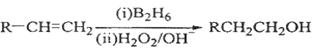
��֪��
��ش��������⣺
��1����״����11.2 L��A�������г��ȼ�տ�������88 g CO2��45 g H2O����A���ӽṹ����3��������A�Ľṹ��ʽΪ ��
��2��B��C��Ϊһ�ȴ�����D������(ϵͳ����)Ϊ ��
��3���ڴ���������1 mol F��2 mol H2��Ӧ������3��������1��������F�Ľṹ��ʽ�� ��
��4����Ӧ�ٵķ�Ӧ������ ��
��5����Ӧ�ڵĻ�ѧ����ʽΪ ��
��6��д��������G������ͬ�����ŵķ�����ͬ���칹��Ľṹ��ʽ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
����ѧ����ѡ��5���л���ѧ��������15�֣�
������H��������·�ߺϳɣ�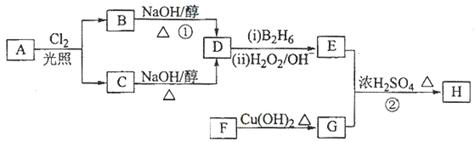
��֪��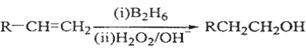
�ش��������⣺
��1��11.2L����״��������A�������г��ȼ�տ�������88gCO2��45gH2O����A���ӽṹ����3��������A�Ľṹ��ʽΪ ��
��2��B��C��Ϊһ�ȴ�����D�����ƣ�ϵͳ������Ϊ ��
��3���ڴ���������1molF��2molH2��Ӧ������3��������1��������F�Ľṹ��ʽ
�� ��
��4����Ӧ�ٵķ�Ӧ������ ��
��5����Ӧ�ڵĻ�ѧ����ʽΪ ��
��6��д��������G������ͬ�����ŵķ�����ͬ���칹��Ľṹ��ʽ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
(12��)����ѧ�����л���ѧ������
ij�л�������K�ĺϳ�·�����£���
��֪��
III��E�ĺ˴Ź�������ͼ��ֻ��һ�����շ塣
��ش��������⣺
��1��A�ķ���ʽΪ_______��C��D�ķ�Ӧ����Ϊ_______��
��2������E��G���õ��Լ���NaOH��Һ�⣬����Ҫ���Լ���_______�����Լ����ƣ���
��3����������������C��ͬ���칹�干��_______�֡�
��������ˮ�����ӳɷ�Ӧ����ʹ�Ȼ�����Һ����ɫ
�۱�����������ȡ�������ܷ���������Ӧ
��4��J��һ�������¿��Է����ۺϷ�Ӧ�õ�һ�ָ߾���ø߾���Ľṹ��ʽΪ____ ____________________��
��5��D+J��K�Ļ�ѧ����ʽΪ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��14�֣�������������������E���������Ѫ֢֬�����Ʒ�����Ӧ��ǰ������ϳ�·�ߣ����ַ�Ӧ������ȥ�����£�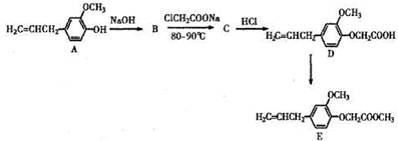
��1��B ��C�ķ�Ӧ������ ��B�Ľṹ��ʽ�� ����2��D�к��������ŵ��������ѻ��� ��
��3����д��ClCH2COONa���ữ������ͬʱ��������������ͬ���칹��ṹ��ʽ
���ܷ���������Ӧ ����������
��4����D�ϳ�E�Ļ�ѧ����ʽ�� ��
��5�����й���A��˵����ȷ���� ��
| A��1molA��ȫȼ������12molO2 | B����ʹ����KMnO4��Һ��ɫ |
| C������NaHCO3��Ӧ | D���������巢���ӳɷ�Ӧ���ܷ���ȡ����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
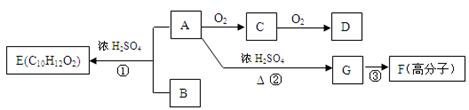
(18��)A��B��һ�������¿ɺϳɷ����廯����E��G�ڱ�״���������壬������µ��ܶ�Ϊ1��25g/L�������ʼ��ת����ϵ������ʾ��

 ��ش��������⣺
��ش��������⣺ ��1��G�еĹ������� (�û�ѧʽ��ʾ)��D�еĹ����������� ��
��1��G�еĹ������� (�û�ѧʽ��ʾ)��D�еĹ����������� ��
F�Ľṹ��ʽ �� ��2��ָ����Ӧ���ͣ��� ���� ���� ��
��2��ָ����Ӧ���ͣ��� ���� ���� �� ��3����������������B��ͬ���칹���� �֡�
��3����������������B��ͬ���칹���� �֡�
����FeCl3��Һ����ɫ ���ܷ���������Ӧ �۱�����ֻ������ȡ���� ��4��д����ѧ����ʽ��A��C ��
��4��д����ѧ����ʽ��A��C �� C�����Ƶ�������ͭ����Һ��Ӧ�� ��
C�����Ƶ�������ͭ����Һ��Ӧ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
���ɽ����ٴ���ż����Ӧ�ǽ������л��ϳɵ��ȵ�֮һ���練Ӧ�٣�
������II�������ºϳ�·��ã�
��1��������I���������ŵ�����Ϊ ��������II�ķ���ʽΪ ��
��2��������IV�Ľṹ��ʽΪ ��ijͬѧ�������辭��Ӧ�ڡ��ۡ��ܺ͢ݣ�ֱ��������KMnO4��Һ�Ϳɽ�������III����Ϊ������VII�����������Բ��������������� ��
��3��������VII�ж���ͬ���칹�壬��д��һ�ַ�������Ҫ��Ľṹ��ʽ ��
i��������������ȡ����
ii��1 mol �����ʷ���������Ӧ������4 mol Ag
��4����Ӧ�Ļ�ѧ����ʽΪ ����ע��������
��5�������� �뻯����
�뻯���� ��һ�������°����ʵ���֮��1��2�ɷ������Ʒ�Ӧ�ٵķ�Ӧ����д�������Ľṹ��ʽ ��
��һ�������°����ʵ���֮��1��2�ɷ������Ʒ�Ӧ�ٵķ�Ӧ����д�������Ľṹ��ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��12�֣����ǻ���Ƥ���Ǻϳ��㾫����Ҫԭ�ϣ���Ϊ�ϳ����ǻ���Ƥ���·��֮һ
��֪��
�Իش��������⣺
��1��������II�Ľṹ��ʽΪ��
��2��������II��������III���л���Ӧ����
��3��������III��������Һ�з�����Ӧ��ѧ����ʽ
��4���л���XΪ������IV��ͬ���칹�壬��֪�л���X�������ص㣺���DZ��Ķ�λȡ���������NaHCO3��Ӧ�ų����壬���ܷ���������Ӧ����д��������X�Ľṹ��ʽ
��5������˵����ȷ���ǣ� ��
| A��������I���Ȼ�����Һ����ɫ | B��������II����NaHCO3��Һ��Ӧ |
| C��1mol������IV��ȫȼ������9��5molO2 | D��1mol������III����3 mol H2��Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com