
(��)���в����ᵼ��ʵ����ƫ�ߵ���________
A������һ�����ʵ���Ũ�ȵ�������Һʱ������ҡ�Ⱥ���Һ����ڿ̶��ߣ�
B��������һ�����ʵ���Ũ����Һʱ���á�10 mL����Ͳ��ȡ5.0 mLҺ������ʱ���Ӷ���
C������ƽ����20.5 gij���ʣ������ҩƷ��λ�÷ŷ�������ҩƷ������
D��10���������90�������������������50����������Һ
E������һ�����ʵ���Ũ����Һʱ������ʱ���Ӷ�����������Һ��Ũ��
(��)����������������Ʒ��
a������̨(����Ȧ������)
b����ƿc���ζ���
d���ձ�(����)
e��������
f����ͷ�ι�
g��������ƽ(������)
h����ֽ
i����Ͳ
j��©��
k���¶ȼƣ�
���������Լ���
A��NaOH����
B��̼������Һ
C���Ȼ�þ��Һ
D������ˮ
����գ�
(1)��ͼ�ֱ����¶ȼơ���Ͳ���ζ��ܵ�һ���֣������жϼ�����(���߿̶�)����ȷ����________
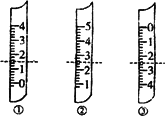
A��������Ͳ������Ϊ1.5 mL
B��������Ͳ������Ϊ2.5 mL
C�����ǵζ��ܣ�����Ϊ2.50 mL
D�������¶ȼƣ�������2.5��
(2)����һ�����ʵ���Ũ�ȵ�����������Һʱ����ȱ�ٵ�������________
(3)��ȥMg(OH)2�л��е�����Ca(OH)2�������õ��Լ��ǣ�________(ѡ�����)������������________��________ϴ�ӣ��������������������������Ʒ�õ�����________(��������������Ʒ��ѡ��������Ӧ���)��
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д� ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
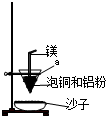 ��ҵ��ұ����ͭ��mCu2O?nFeS���ɵõ���ͭ�����Դ�ͭΪԭ���Ʊ�����ͭ���壮
��ҵ��ұ����ͭ��mCu2O?nFeS���ɵõ���ͭ�����Դ�ͭΪԭ���Ʊ�����ͭ���壮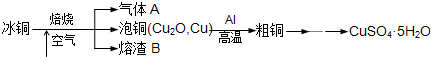
| ||
| ||
| ��� |
| 5bc |
| 4a |
| 5bc |
| 4a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
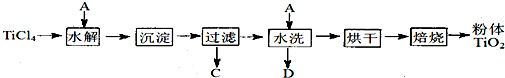

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���к��Ȳⶨʵ���У���ͭ�ƻ��ν��������滷�β���������������к��ȵ���ֵ | B���к͵ζ��ⶨ������ҺŨ�ȣ���ȡ20.00mL����������Һ�ĵζ���δ�ô���������Һ��ϴ | C����������ƽ����10.5gij���ʣ������ҩƷ��λ�÷ŷ�������ҩƷ������ | D������һ�����ʵ���Ũ����Һʱ������Ͳ��ȡŨ��Һ�����ʱ���Ӷ�����������Һ��Ũ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com