
��8�֣�ijͬѧ�����в�������500 mL 0.2 mol��L��1 KCl��Һ����ش��й����⡣
| ʵ�鲽�� | �й����� |
| �ټ�������KCl������ | ��ҪKCl������Ϊ________g(����С�����һλ) |
| �ڳ���KCl���� | ������Ҫ�õ�����Ҫ������________________ |
| �۽�KCl����100 mL�ձ��У�����������ˮ | Ϊ�˼ӿ��ܽ����ʣ����Բ�ȡ��Щ��ʩ�� ________________ |
| �ܽ��ձ�����Һת����500 mL����ƿ�� | Ϊ�˷�ֹ��Һ������Ӧ��ȡʲô��ʩ�� __________________ |
| ��������ƿ�м�����ˮ���̶��� | �ڽ��д˲���ʱ����ˮ����̶���1 cm��2 cm��Ӧ��β�����____________________ |
��7.5����������ƽ��ҩ�ס��۽���(���ʵ�����)�����ò���������������
�ݸ��ý�ͷ�ιܼ�ˮ����Һ����̶�������
��1������ƿ�Ƿ�©ˮ����2��ƫ�ͣ�3����������
������������� ��������֪��500mL 0.2mol?L-1KCl��Һ�к��������Ȼ���0.1mol����Ҫ�Ȼ��ص�����Ϊ��74.5g/mol��0.1mol=7.45��7.5g������7.5g�Ȼ��أ�����ʹ��������ƽ���г�����Ϊ�˼����ܽ⣬��Ҫʹ�ò��������н��裻ת����Һʱ����Ҫʹ�ò�����������Ŀ���DZ���Һ����������ƿ��ߣ�����ʱ����ֱ�Ӽ�����ˮ������ƿ�̶���1-2cm�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������У���1����ʹ������ƿ����һ�����ʵ���Ũ�ȵ���Һʱ������������ƿ�Ƿ�©ˮ����2��δ��ϴ���ձ���������ϴҺת��������ƿ���������Ƶ���Һ�����ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ���3�������Тݲ�����ʱ������ˮ�����̶��ߣ��ᵼ�����Ƶ���ҺŨ��ƫ�ͣ��˴�����ʧ�ܣ���Ҫ�����������ơ�
���㣺����������һ�����ʵ���Ũ�ȵ���Һ��������������


 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��NAΪ�����ӵ�������ֵ������˵����ȷ����
| A��1L0.5mol��L��1 ��(NH4)2SO4��Һ�к�NH4+��ΪNA |
| B�����³�ѹ�£�1molCH4�к���4NA��C��H�� |
| C�����³�ѹ�£�48g O3����ԭ����Ϊ2NA�����ԭ��������O 16�� |
| D����״���£�22.4LC6H6(��)�ķ�����ĿΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��һ��������Ϊ�ص�KOH��Һ������m gˮ����������ǡ�ñ�Ϊ3�أ����ΪVL(��Һ����������)����Ũ������Һ�����ʵ���Ũ��Ϊ�� ��
A�� mol��L-1 mol��L-1 | B�� mol��L-1 mol��L-1 |
C�� mol��L-1 mol��L-1 | D�� mol��L-1 mol��L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ѧ������ѧ�û�ѧ֪ʶ����Ҫ�����������йػ�ѧ�����ʾ��ȷ���ǣ� ��
���õ���ʽ��ʾHCl���γɹ��̣�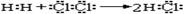
��MgCl2�ĵ���ʽ��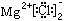
��������Ϊ133��������Ϊ78���ԭ�ӣ�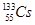
����ϩ������ṹ��ʽ����Ϊ��CH2CH2��C2H4O2
��S2-�Ľṹʾ��ͼ��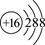
| A���٢ڢۢܢ� | B���ܢ� | C���ۢ� | D���ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A���弫������ˮ������Է�������Ϊa����0�桢1��01��105Paʱ������䱥����Һ�����ʵ���Ũ��Ϊb mol/L������Һ���ܶ�Ϊc g/cm3����
��1��A������Һ�����ʵ�����������
��c%��
��2��0�桢1��01��105��ʱ��1���ˮ�п��ܽ���������A����?
��V��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ƽ�����ʢ��ǿ��ԭ���£�N2H4����ǿ������Һ̬˫��ˮ�������ǻ�Ϸ�Ӧʱ������������������ˮ���������ų������ȡ���֪0.4molҺ̬��������Һ̬˫��ˮ��Ӧ�����ɵ�����ˮ�������ų�256.652KJ��������
��1��д����������ĵ���ʽ ��
��2���÷�Ӧ���Ȼ�ѧ����ʽΪ ��
1mol����ȫ��Ӧת�Ƶ����� ��
��3���˷�Ӧ���ڻ���ƽ������ͷŴ����ȺͿ��ٲ������������⣬����һ���ܴ���ŵ��� ��
��4������֪H2O(l)==H2O(g)����H = +44kJ?mol-1����16gҺ̬����Һ̬˫��ˮ��Ӧ����Һ̬ˮʱ�ų��������� kJ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��CO2ͨ��NaOH��Һ�У����ò�����ͨ���CO2�����ʵ����IJ�ͬ����ͬ�����������ա�
��1��250 mL 1 mol/L��NaOH��Һ����������CO2 L����״���£���
��2����250 mL 2 mol/L��NaOH��Һ��ͨ��һ����CO2����Һ����4.4 g����������Һ���ɣ��������ù����и��ɷֵ����ʵ�����
��δ֪Ũ�ȡ����ΪV L�� NaOH��Һ�л���ͨ��һ���������״���£���CO2����ַ�Ӧ���ڼ�ѹ���µ�������������Һ���õ���ɫ���塣
��3������Ӧ��CO2��NaOH����ʣ�࣬��Ӧ������Һ�м�������ij���ʯ��ˮ����m1 g ��ɫ������
�ٸ����������ݣ��ô���ʽ��ʾCO2�����V(CO2)�� ��
�ڸ����������ݣ����������NaOH��Һ��Ũ�ȷ�Χ��
��4��д��ȷ��NaOHŨ�ȵ�ʵ�鲽�裬���ô���ʽ��ʾNaOH��Һ��Ũ�ȡ������ʵ�鷽��ʱ��ֻ���������ṩ��CO2��NaOH��Һ������ʹ��������ѧ�Լ�����
ʵ�鲽�裺 ��c(NaOH)�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��״����336 L��NH3����1 Lˮ�У�������Һ����������Ϊ________��������Һ���ܶ�Ϊa g/cm3�������ʵ���Ũ��Ϊ________����������ˮȫ��ת��ΪNH4Cl������4 mol��L��1����������Ϊ________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com