
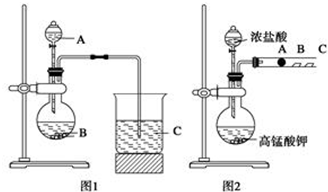
���� ��1��ʵ��ǰ�ζ��ܱ�����в�©���ζ���װҺ���������ݣ��ٵ��㣬Һ�浽0�̶Ȼ�0�̶����£��ζ�ʱ���۾�Ӧע����ƿ����Һ����ɫ�仯�����ж��ζ��յ㣻���ᷴӦ��ϣ��������һ��KMnO4��Һ����Һ��Ϊ��ɫ����ɫ30s�ڲ���ȥ��˵���ζ����յ㣻
��2������c�����⣩=$\frac{c��������V������}{V�����⣩}$�жϲ��������������������Ӱ�죮
��� �⣺��1���ζ�����ʹ��֮ǰ��������еIJ����Ǽ���Ƿ�©ˮ��Ȼ����ˮϴ�ӣ��ô�װҺ��ϴ�����ڵζ�����װ����Һ��Ҫ�����ݣ��ٵ�Һ�浽0�̶Ȼ�0�̶����£���������еζ����ζ�ʱ���۾�ע����ƿ����Һ��ɫ�ı仯���õζ��ﵽ�ζ��յ�ʱ������Ϊ��Һ����ɫǡ�ñ�Ϊdz�Ϻ�ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ������Ƿ�©ˮ�������ݣ�ע����ƿ����Һ��ɫ�ı仯����Һ����ɫǡ�ñ�Ϊdz�Ϻ�ɫ���Ұ�����ڲ���ɫ��
��2��A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱ���������Һ����������V������ƫС������c�����⣩=$\frac{c��������V������}{V�����⣩}$�жϣ��ⶨ���ƫ�ͣ���A����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ����������ݣ����V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$�жϣ��ⶨ���ƫ�ߣ���B��ȷ��
C��ʢװ���������Һ�ĵζ���������ˮ��ϴ����δ�ø��������Һ��ϴ����Һ��Ũ��ƫС�����V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$�жϣ��ⶨ���ƫ�ߣ���C��ȷ��
D��ʢװ������Һ����ƿ������ˮ��ϴ����δ�ò�����Һ��ϴ����ȷ�IJ�����V���������䣬����c�����⣩=$\frac{c��������V������}{V�����⣩}$�жϣ��ⶨ������䣬��D����
E���μӸ��������Һ���죬δ������տ�����Һ��ɫ������ֹͣ�ζ������V������ƫС������c�����⣩=$\frac{c��������V������}{V�����⣩}$�жϣ��ⶨ���ƫ�ͣ���E����
��ѡBC��
���� ������Ҫ������������ԭ�ζ��IJ������衢�ζ��ܵ�ʹ�á����������Ѷ��еȣ����յζ���ԭ���ǽ���Ĺؼ���


 �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д� ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
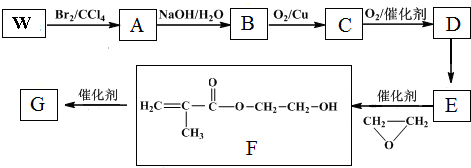
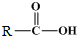 +
+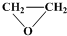 $\stackrel{����}{��}$
$\stackrel{����}{��}$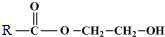
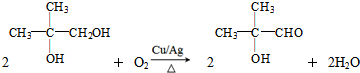 ��
�� ��
���鿴�𰸺ͽ���>>
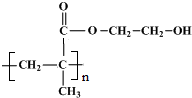
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �ζ����� | ������� | NaOH��Һ���������mL�� | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.00 | 18.10 |
| 2 | 20.00 | 0.00 | 16.30 |
| 3 | 20.00 | 0.00 | 16.22 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
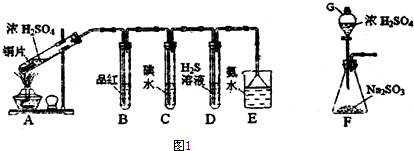
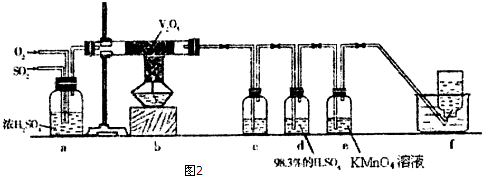
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �����ӵ�һ�ȴ�����2�֣�
�����ӵ�һ�ȴ�����2�֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
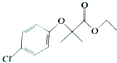 �����ٴ���һ�ֽ�֬��Ѫ˨ҩ�����һ���ϳ�·�����£�
�����ٴ���һ�ֽ�֬��Ѫ˨ҩ�����һ���ϳ�·�����£�

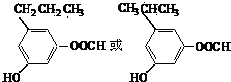 ��
�� ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com