
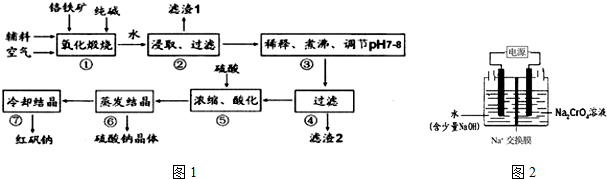
���� ��������Ҫ�ɷ�ΪFeO•Cr2O3��������Al2O3��SiO2�����ʣ�����̼�������գ�������Na2CrO4��Fe2O3��NaAlO2��Na2SiO3�ȣ�����ˮ��ȡ���õ�����1ΪFe2O3�����˺���Һϡ�͡���С�����pH7��8�õ�����2ΪAl��OH��3��H2SiO3�����˺���Һ�ữ�������˷�Ӧ2Na2CrO4+H2SO4?Na2SO4+Na2Cr2O7+H2O��������Na2Cr2O7��Na2SO4�������ᾧ�õ������ƣ���ȴ�ᾧ�õ�Na2Cr2O7•2H2O��
��1������Al2O3�ڢ���ת������NaAlO2��
��2����Һ�к���NaAlO2��Na2SiO3�ȣ���Ϊǿ�������Σ�������У��ٽ�ˮ�⣬������pH���ͣ�H+��ˮ�����ɵ��������������ܽ⣬����Al3+��Na2CrO4���Է��룻
��3�����ữʱ�����ķ�ӦΪ��2CrO42-+2H+?Cr2O72-+H2O�������غ��й�ϵʽ��2Cr��2CrO42-��Cr2O72-����ʽ�����n��Cr2O72-����n��CrO42-��ʣ�࣬��������ữ��������Һ��c��Cr2O72-����
�ڸ���2CrO42-+2H+?Cr2O72-+H2O���ͼʾװ�ã���Ϊ���Ե缫�����Ҳ�缫���Na2CrO4��Һ��ȡNa2Cr2O7��˵���ڸõ缫�����������ӵ�Դ���������缫��ӦʽΪ2H2O-4e-=O2+4H�������缫��Ӧ2H++2e-=H2�����ݴ˼��㣮
��� �⣺��������Ҫ�ɷ�ΪFeO•Cr2O3��������Al2O3��SiO2�����ʣ�����̼�������գ�������Na2CrO4��Fe2O3��NaAlO2��Na2SiO3�ȣ�����ˮ��ȡ���õ�����1ΪFe2O3�����˺���Һϡ�͡���С�����pH7��8�õ�����2ΪAl��OH��3��H2SiO3�����˺���Һ�ữ�������˷�Ӧ2Na2CrO4+H2SO4?Na2SO4+Na2Cr2O7+H2O��������Na2Cr2O7��Na2SO4�������ᾧ�õ������ƣ���ȴ�ᾧ�õ�Na2Cr2O7•2H2O��
��1������Al2O3�ڢ���ת���Ļ�ѧ��Ӧ����ʽΪAl2O3+Na2CO3$\frac{\underline{\;����\;}}{\;}$2NaAlO2+CO2����
�ʴ�Ϊ��Al2O3+Na2CO3$\frac{\underline{\;����\;}}{\;}$2NaAlO2+CO2����
��2����Һ�к���NaAlO2��Na2SiO3�ȣ���Ϊǿ�������Σ�������У��ٽ�ˮ��ƽ��AlO2-+2H2O?Al��OH��3+OH-��SiO32-+2H2O?H2SiO3+2OH-�����ƶ��������������������������������pH���ͣ�H+��ˮ�����ɵ��������������ܽ⣬����Al3+��Na2CrO4���Է��룬
�ʴ�Ϊ��ˮ�����ȣ���дٽ�ˮ��ƽ��AlO2-+2H2O?Al��OH��3+OH-��SiO32-+2H2O?H2SiO3+2OH-�����ƶ�������������������������� H+��ˮ�����ɵ��������������ܽ⣬����Al3+��Na2CrO4���Է��룻
��3�����ữʱ�����ķ�ӦΪ��2CrO42-+2H+?Cr2O72-+H2O����1L�ữ��������Һ�к���Ԫ�ص�����Ϊ28.6g��CrO42-��$\frac{10}{11}$ת��ΪCr2O72-��˵����Ԫ����$\frac{10}{11}$ת��ΪCr2O72-
�����غ��й�ϵʽ��2Cr��2CrO42-��Cr2O72-
2 1
$\frac{28.6g��\frac{10}{11}}{52g/mol}$ n��Cr2O72-��
��n��Cr2O72-��=0.25mol��
���ữ��������Һ��c��Cr2O72-��=$\frac{n}{V}$=0.25mol•L-1��
�ʴ�Ϊ��0.25mol•L-1��
�ڸ���2CrO42-+2H+?Cr2O72-+H2O���ͼʾװ�ã���Ϊ���Ե缫�����Na2CrO4��Һ��ȡNa2Cr2O7����Ҫͨ���������H+�����Һ�����ԣ�˵���ڸõ缫�����������ӵ�Դ���������缫��ӦʽΪ2H2O-4e-=O2+4H+��ͨ����ɺ���ת��0.1mol���ӣ������缫��Ӧ��2H2O-4e-=O2+4H+��4OH--4e-=2H2O+O2���������缫��Ӧ2H++2e-=H2����2CrO42-+2H+=Cr2O72-+H2O����ԭ����Һ������ͬΪm��������Һ�е���غ㣬������������������������=m-0.5mol��2g/mol+1mol��23g/mol-��m-0.25mol��32g/mol-1mol��23g/mol��
=53g��
�ʴ�Ϊ��2H2O-4e-=O2+4H+��4OH--4e-=2H2O+O2����53��
���� ���⿼���������Ʊ����̺ͷ����ķ����жϣ��������ʵ�Ӧ�ã�Ϊ��Ƶ���㣬������ѧ���ķ���������ʵ�������Ŀ��飬ע����������Ϣ�ķ������⣬���������ע������ͻ������������ǽ���ؼ�����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
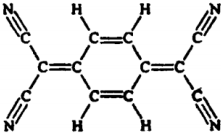
| A�� | ���������еĵ�ԭ����ͬһƽ���� | B�� | ���ڷ����� | ||
| C�� | ����ʽΪC12H4N4 | D�� | ������������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ̼���� | B�� | �Ҵ� | C�� | �Ȼ��� | D�� | һ����̼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2O2���壨ϡ���ᣩ | B�� | FeCl2��Һ��KSCN��Һ�� | ||
| C�� | KI���壨������Һ�� | D�� | NaOH��Һ�����ᣩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����������Ԫ��A��B��C��D��E��Ԫ�����ڱ��е�λ����ͼ��ʾ������AΪ�ؿ��к�����ߵĽ���Ԫ�أ�
����������Ԫ��A��B��C��D��E��Ԫ�����ڱ��е�λ����ͼ��ʾ������AΪ�ؿ��к�����ߵĽ���Ԫ�أ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
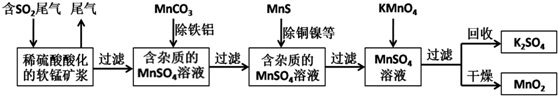
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ���еļױ�����ˮ�� | B�� | �Ҵ��е�ˮ������CaO�� | ||
| C�� | ��ȩ�е����ᣨNaOH�� | D�� | ���������е����ᣨ����NaHCO3��Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com