

���� ��1��8CuFeS2+21O2$\frac{\underline{\;����\;}}{\;}$8Cu+4FeO+2Fe2O3+16SO2�У�FeԪ�صĻ��ϼ����ߣ�Cu��OԪ�صĻ��ϼ۽��ͣ�
��2��ұ��ͭ����Ҫ���̣���ͭ����ʯӢɰ��������ϱ��յõ���ͭ��mCu2S•nFeS��������ʯӢɰͨ��������գ�����Cu2O��Cu����������AΪ������������ΪSiO2�ȣ������������������ܽ���˳�ȥ���������SiO2�ͺ�����Ԫ�ص�������ӵ�������Һ�����ù�������������Һ���з���õ�������������������ϴ�����յõ�����������ͭ��Alͨ�����ȷ�Ӧ�õ���ͭ�������õ��õ���ͭ��
��3��ͭ����ϡH2SO4��H2O2���Һ��������ͭ����E�����û���Ӧ����ͭ���ʺ���һ������X��X��ϡH2SO4��H2O2���Һ���ɵ�Y��KSCN��Һ��Ѫ��ɫ����Ϊ�����ӣ���EΪFe��XΪFeSO4��YΪFe2��SO4��3��ZΪFe��OH��3���ݴ˷�������
��� �⣺��1��8CuFeS2+21O2$\frac{\underline{\;����\;}}{\;}$8Cu+4FeO+2Fe2O3+16SO2�У�FeԪ�صĻ��ϼ����ߣ�Cu��OԪ�صĻ��ϼ۽��ͣ���Cu��OԪ�ر���ԭ��
�ʴ�Ϊ��Cu��O��
��2����������ͼת����֪������A�еĴ�����Ⱦ����Ҫ�Ƕ�������ѡ���Լ����ն��������ܲ����µ���Ⱦ���壬a��ŨH2SO4�������ն������ʴ���
b��ϡHNO3�������ն�����������NO��Ⱦ�������ʴ���
c��NaOH��Һ���������Ӧ�����������ƣ�����ȷ��
�ʴ�Ϊ��c��
����ϡHNO3��������B���������������ӣ�������Ϊ�����ӣ�Ҳ��ʹKSCN��Һ��Ѫ��ɫ���ʸý��۲���ȷ��
�ʴ�Ϊ���ý��۲���ȷ��ϡHNO3��ǿ�����ԣ��������ļ�̬Ϊ+2�ۣ�������Ϊ+3��ͬ����ʹKSCN��Һ��Ѫ��ɫ��
��3���������̿�֪��Cu����ϡH2SO4��H2O2���Һ��������ͭ�����������ӷ���ʽΪ��Cu+H2O2+2H+�TCu2++2H2O��
�ʴ�Ϊ��Cu+H2O2+2H+�TCu2++2H2O��
���ɷ�����֪ZΪFe��OH��3��EΪFe��Cl2��Z����ΪK2FeO4����Ԫ�ػ��ϼ����ߣ�����Ԫ�ػ��ϼ۽���Ϊ-1�ۣ������ķ�ӦΪ��10KOH+3Cl2+2Fe��OH��3�T2K2FeO4+6KCl+8H2O��
�ʴ�Ϊ��10KOH+3Cl2+2Fe��OH��3�T2K2FeO4+6KCl+8H2O��
�۶���0.1mol•L-1 CuSO4��Һ��
A�������£�ͭ���ӷ���ˮ�⣬Cu2++2H2O=Cu��OH��2+2H+��pH��7������
B��ͭ���ӷ���ˮ�⣬c��SO42-����c��Cu2+����c��H+����c��OH-��������
C�����ݵ���غ㣬c��H+��+2c��Cu2+��=2c��SO42-��+c��OH-������ȷ��
�ʴ�Ϊ��C��
���� ����Χ��ͭԪ�ص��й����ʿ�����Ԫ�ػ���������ʡ����ʵ��Ʊ������Ӽ��顢�����ƶϡ���Һ�����ӵ�ˮ����غ��֪ʶ�㣬����ƴ������Ŀ���������ʵ�������ͻ�Ƶ㣬�Ѷ��еȣ�


 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ��һ��10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�ڱ�״���£�1���ˮ�ܽ�672���������������Һ�ܶ�Ϊ0.9g��cm��3�����ְ�ˮ�����ʵ���Ũ�Ⱥ����ʵ����������ֱ�Ϊ( ��
A��17.9mol��L��1 34.7% B��20.4mol��L��1 33.8%
C��17.9mol��L��1 33.8% D��20.4mol��L��1 34.7%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
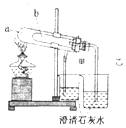 ij�о���ѧϰС�������ʦ��ѧ�����ʵ���̼���ƺ�̼�����Ƶ����ʽ����о���
ij�о���ѧϰС�������ʦ��ѧ�����ʵ���̼���ƺ�̼�����Ƶ����ʽ����о����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | N2��NH3 | B�� | Al2O3��Al��OH��3 | C�� | Fe��FeCl2 | D�� | SiO2��CaSiO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ��һ��10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����6.02��1023��Oԭ�ӵ�H3PO4�����ʵ����ǣ� ��
A��1 mol B��0.5 mot C��0.25 mol D��0.125 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ��һ��10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���ҳ�����ͼʾ����ȷ��ʵ������� ��
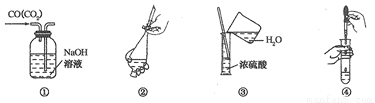
A. �ٳ�ȥCO�е�CO2 B. ����ȡʱ���Һ
C. ��ϡ��Ũ���� D. ���Թ��еμ�Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ�߶���10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪��X2(g)+Y2(g) 2Z(g)��X2��Y2��Z����ʼŨ�ȷֱ�Ϊ0.1mol��L��1��0.3 mol��L��1��0.2 mol��L��1����һ�������£�����Ӧ�ﵽƽ��ʱ�������ʵ�Ũ�ȿ�����
2Z(g)��X2��Y2��Z����ʼŨ�ȷֱ�Ϊ0.1mol��L��1��0.3 mol��L��1��0.2 mol��L��1����һ�������£�����Ӧ�ﵽƽ��ʱ�������ʵ�Ũ�ȿ�����
A��ZΪ0.4mol��L��1
B��X2Ϊ0.2 mol��L��1
C��Y2Ϊ0.4 mol��L��1
D��ZΪ0.3 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ�߶��Ͻ�ѧ�ʼ컯ѧ�Ծ��������棩 ���ͣ�ѡ����
��������ͬʱ�������Ӽ������ۼ�����λ���Ļ�������
A��NH4Cl B�� Na2O2 C��H3O+ D��MgO
Na2O2 C��H3O+ D��MgO
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com