

 2Fe2O3+4SO3������������������ԭ�����������������������ͻ�ԭ�������ʵ���֮��Ϊ1��4��
2Fe2O3+4SO3������������������ԭ�����������������������ͻ�ԭ�������ʵ���֮��Ϊ1��4��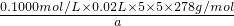 ��100%=
��100%=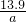 ���ʴ�Ϊ��
���ʴ�Ϊ��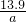 ��
��

 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 13.9 |
| a |
| 13.9 |
| a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 13.9 |
| a |
| 13.9 |
| a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ϻ��з�����������ѧ����ĩ��ѧ�������л�ѧ�Ծ��������棩 ���ͣ������
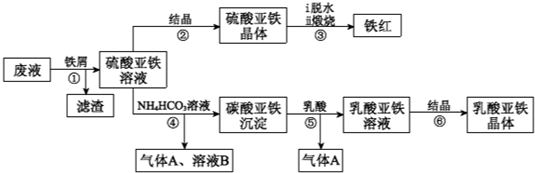
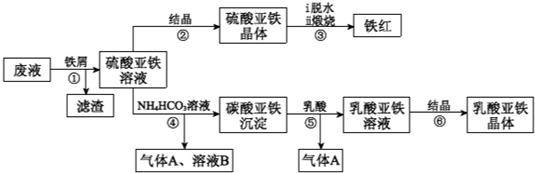
������ⷨ���Ѱ۲����ķ�Һ[���д���FeSO4��H2SO4������Fe2(SO4)3��TiOSO4]����������Ͳ�Ѫ�������������������������£�

��֪��TiOSO4������ˮ����ˮ�п��Ե���ΪTiO2+��SO42������ش�
��1��������з�������������Һ�������IJ��������õIJ��������� ��
����ڵõ�������������IJ���Ϊ����Ũ���� ��
��1������ܵ����ӷ���ʽ�� ��
��1������ޱ������һ������նȣ�ԭ��������������ˮ�Լ� ��
��1�����������ڿ�������������������������÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ ��
��1����ƽ���ƶ���ԭ�����Ͳ�����м������ܵõ�����������ԭ�� ��
��1��Ϊ�ⶨ����������þ�����FeSO4��7H2O������������ȡ������Ʒa g������ϡ�������100��00 mL��Һ��ȡ��20��00 mL��Һ����KMnO4��Һ�ζ���������KMnO4����Ӧ����������0��1000 mol•L-1 KMnO4��Һ20��00 mL�����þ�����FeSO4��7H2O����������Ϊ����a��ʾ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�갲��ʡ�Ϸ��и��������ν�ѧ����������ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
������ⷨ���Ѱ۲����ķ�Һ[���д���FeSO4��H2SO4������Fe2(SO4)3��TiOSO4 ]���������������Ͳ�Ѫ�������������������������£�

��ش�
��1��������з��������������_______������ޱ������һ������նȣ�ԭ����______________
��2����Һ�е�TiOSO4�ڲ������ˮ����������(��Ҫ�ɷ�ΪTiO2•xH2O)�Ļ�ѧ����ʽΪ_________
_ ������ܵ����ӷ���ʽΪ____________________________________��
��3����ƽ���ƶ�ԭ�����Ͳ�����м������ܵõ�����������ԭ��______________________________��
��4����ƽ���Ը��������Һ������������Һ��Ӧ�����ӷ���ʽ��
_____ Fe2+ + _____ MnO4�� + _____ H+ = _____Fe3+ +_____ Mn2+ +_____
ȡ��������þ�����Ʒa g,����ϡ�������100.00 mL��Һ��ȡ��20. 00 mL��Һ,��KMnO4��Һ�ζ���������KMnO4����Ӧ����������0.1000 mol��L��1 KMnO4��Һ20.00mL�������þ�����FeSO4 • 7H2O����������Ϊ���Ժ�a��ʽ�ӱ�ʾ) _____________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com