
| ������NaOH��Һ�������� | ������NaCl��ҺŨ�ȣ�g/L�� | |
| ����Һ | 0.30 | 310 |
| ���� | 0.32 | 210 |
���� ��1������n=$\frac{V}{{V}_{m}}$������������ʵ������������������������������������ʵ������ٽ�Ϧ�=$\frac{m}{V}$�����ܶȣ��缫��Ӧ����ʽΪ��2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+Cl2��+H2�����������ϲ����������Ƶ����ʵ������������ʵ�����2����
��2����Һ��NaOH��������Ϊ���õ���NaOH��������Һ������NaOH������������������NaOH���������������ٵ�NaCl���ʵ������������ɵ�NaOH���ʵ�����ȣ����������μӷ�ӦNaCl���������ٽ������NaClŨ�ȱ仯���㣻
����40%����Һ����Ʒ�е�NaOH��������ϡ����NaOH�������䣬����ϡ�ͺ���Һ�������ټ�����Ҫˮ������������������Ҫ���봿ˮ�����
��3�������������������Ʒ�Ӧ�Ʊ�Ưˮ����NaCl���ɣ����pH=12������ˮ�⣩��˵����NaOHʣ�࣬���Լ�����Һ���������Ƶ�Ũ�ȣ����������������Ƶ����ʵ���������������Ƶ����ʵ��������ݵ���ת���غ����NaCl���ʵ������������վ��������жϾ������Ƿ���ˮ�����У�����ˮ�����ʵ�������������ʵ����ʵ���֮��ȷ����ѧʽ��
��� �⣺��1������V=nVm����������485.92m3�����ʵ�����$\frac{485920L}{22.4L/mol}$=2.169��104mol��Cl2���������Ϊ0.985������ΪO2�������������ܶ�Ϊ��$\frac{485920L}{22.4L/mol}$��0.985��71g/mol+$\frac{485920L}{22.4L/mol}$��0.015��32g/mol����485920L=3.144g/L���������ϲ����������Ƶ����ʵ������������ʵ�����2������2.169��104mol��0.985��2=4.273��104mol��
�ʴ�Ϊ��2.169��104mol��3.144g/L��4.273��104mol��
��2����Һ��NaOH��������Ϊ���õ���NaOH����������NaOHΪ90.416kg��0.32-52.000kg��0.30��
�������ٵ�NaCl���ʵ������������ɵ�NaOH���ʵ�����ȣ�
����������NaCl����������90.416kg��0.32-52.000kg��0.30����$\frac{58.5}{40}$��
��������ÿСʱ����NaCl��Һ Ϊx m3�����������ٵ�NaCl����Ϊ��310-210��kg/m3��x m3��
���ԣ���90.416kg��0.32-52.000kg��0.30����$\frac{58.5}{40}$=��310-210��kg/m3��x m3��
���x=0.1950
40%����Һ����Ʒ�е�NaOH������Ϊ90.416kg��40%��0.32������һ������ˮ���ﵽ��������Һ��Ũ��Ҫ����ϡ�ͺ���Һ����Ϊ90.416kg��40%��0.32��0.3��
�ʼ���ˮ������Ϊ��90.416kg��40%��0.32��0.3-90.416kg��40%��
�����ˮ�����Ϊ����90.416kg��40%��0.32��0.3-90.416kg��40%����1kg/L=2.411��
�ʴ�Ϊ��0.1950��2.411��
��3�������������������Ʒ�Ӧ�Ʊ�Ưˮ����NaCl���ɣ����pH=12������ˮ�⣩��˵����NaOHʣ�࣬��Һ���������Ƶ�Ũ��Ϊ0.01mol/L����n��NaOH��=0.01mol/L��1L=0.01mol��
NaClO����Ϊ0.3725g���������ʵ���Ϊ$\frac{0.3725g}{74.5g/mol}$=0.005mol��
���ݵ���ת���غ㣬��֪����NaClΪ$\frac{0.005mol��1}{1}$=0.005mol
��m��NaOH��+m��NaClO��+m��NaCl��=0.01mol��40g/mol+0.3725g+0.005mol��58.5g/mol=1.065g��1.335g��
�ʾ����к���ˮ��������Ϊ1.335g-1.065g=0.27g��ˮ�����ʵ���Ϊ$\frac{0.27g}{18g/mol}$=0.015mol��
��n��NaCl����n��NaClO����n��NaOH����n��H2O��=0.005��0.005��0.01��0.15=1��1��2��3��
�ʸþ���Ļ�ѧʽΪNaCl��NaClO��2NaOH��3H2O��
�𣺸þ���Ļ�ѧʽΪNaCl��NaClO��2NaOH��3H2O��
���� ���⿼����ԭ�������㡢���ʵ������㡢�������㣬���ؿ���ѧ�����������������ݵĴ�����������Ŀ��������Ϊ�״���Ŀ���ѶȽϴ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ���¶��£�10mL 0.50mol•L-1NH4Cl��Һ��20mL 0.25mol•L-1NH4C1��Һ��NH4+���ʵ�����ͬ | |
| B�� | 25��ʱ����a mo1•L-l��ˮ��0.01 moI•L-1����������ϣ���Ӧ��ȫʱ��Һ��c��NH4+��=c��C1-�����ú�a�Ĵ���ʽ��ʾ��Ӧ��ȫʱNH3•H2O�ĵ��볣��Kb=$\frac{1{0}^{-9}}{a-0.01}$ | |
| C�� | һ���¶��£���֪0.1 mol•L-1 �Ĵ�����Һ�д��ڵ���ƽ�⣺CH3COOH?CH3COO-+H+���������ռ���Һ��ʹ��Һ��$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$ֵ���� | |
| D�� | �������pH��Ϊ3����HA��HB�ֱ���������п��Ӧ��HA�ų��������࣬˵�����ԣ�HA��HB |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �����������ṹ��ʽ��
�����������ṹ��ʽ�� ��ͬϵ�����������ͬϵ��������ͨʽ�ǣ�������
��ͬϵ�����������ͬϵ��������ͨʽ�ǣ�������| A�� | CnH2n-6��n��11�� | B�� | CnH2n-12��n��10�� | C�� | CnH2n-10��n��10�� | D�� | CnH2n-12��n��10�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
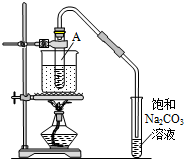 ����ͼ��ʾװ�ã����Թ�A�����3mL�Ҵ���2mL�����ᣬȻ��һ��ҡ����һ�������ؼ���2mLŨ���ᣬ�ټ����������Ƭ���þƾ��Ƽ���10min������ڱ���̼������Һ��Һ����û����ɫ��״Һ�壬���ж���ԭ��ķ���������ǣ�������
����ͼ��ʾװ�ã����Թ�A�����3mL�Ҵ���2mL�����ᣬȻ��һ��ҡ����һ�������ؼ���2mLŨ���ᣬ�ټ����������Ƭ���þƾ��Ƽ���10min������ڱ���̼������Һ��Һ����û����ɫ��״Һ�壬���ж���ԭ��ķ���������ǣ�������| A�� | �Թ�A��û�з�����Ӧ | |
| B�� | ��ԴС��ˮ���࣬ˮԡ�����ٶ�̫�� | |
| C�� | �����������Թ�A�ϲ�ȫ������������ | |
| D�� | ������ȫ���ܽ��ڱ���Na2CO3��Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �����£���0.100 0mol•L-1 NaOH��Һ�ζ�20.00mL 0.100 0mol•L-1 CH3COOH��Һ�ζ�������ͼ��ʾ������˵����ȷ���ǣ�������
�����£���0.100 0mol•L-1 NaOH��Һ�ζ�20.00mL 0.100 0mol•L-1 CH3COOH��Һ�ζ�������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | �����ʾ��Һ�У�c��Na+��=c��CH3COOH��+c��CH3COO-�� | |
| B�� | �����ʾ��Һ�У�c��Na+��=c��CH3COOH��+c��CH3COO-�� | |
| C�� | �����ʾ��Һ�У�c��Na+����c��OH-����c��CH3COO-����c��H+�� | |
| D�� | �ζ������п��ܳ��֣�c��CH3COOH����c��CH3COO-����c��H+����c��Na+����c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
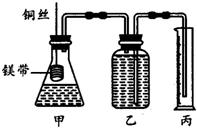 ij�о���ѧϰС��Ϊ��֤����ͬ��ͬѹ�£���ͬŨ����ͬ��������Բ�ͬ��һԪ��������þ����Ӧʱ�����������������ͬ����Ӧ���ʲ�ͬ��ͬʱ�ⶨʵ���������µ�����Ħ���������Ƶļ���ʵ��װ����ͼ��ʾ����ʵ�����Ҫ�����������£�
ij�о���ѧϰС��Ϊ��֤����ͬ��ͬѹ�£���ͬŨ����ͬ��������Բ�ͬ��һԪ��������þ����Ӧʱ�����������������ͬ����Ӧ���ʲ�ͬ��ͬʱ�ⶨʵ���������µ�����Ħ���������Ƶļ���ʵ��װ����ͼ��ʾ����ʵ�����Ҫ�����������£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com