
 ij�о���С�����A-D������װ������й�ʵ��
ij�о���С�����A-D������װ������й�ʵ��

 ��ʵ��һ���ռ�NO���塣
��ʵ��һ���ռ�NO���塣 ��װ��A�ռ�NO���壬��ȷ�IJ����� ������ţ���
��װ��A�ռ�NO���壬��ȷ�IJ����� ������ţ��� a.�Ӣٿڽ���������ˮ������ b.�Ӣٿڽ�����������������
a.�Ӣٿڽ���������ˮ������ b.�Ӣٿڽ����������������� c.�Ӣڿڽ���������ˮ������ d..�Ӣڿڽ�����������������
c.�Ӣڿڽ���������ˮ������ d..�Ӣڿڽ����������������� ��ʵ�����Ϊ��̽����п�������ϵ�п����������
��ʵ�����Ϊ��̽����п�������ϵ�п����������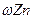 �ͶƲ��ȣ���ѯ��֪п�����ڼZn+2NaOH=Na2ZnO3+H2���ݴˣ���ȡ���ΪS��˫���п���������������顢�Ƶ�����Ϊm1 g���ù����ռ��ˮ���Լ����������ʵ�鷽�����������ʵ�顣
�ͶƲ��ȣ���ѯ��֪п�����ڼZn+2NaOH=Na2ZnO3+H2���ݴˣ���ȡ���ΪS��˫���п���������������顢�Ƶ�����Ϊm1 g���ù����ռ��ˮ���Լ����������ʵ�鷽�����������ʵ�顣 �����ף�ͨ������������Ӧ���ɵ����������ʵ��̽��ľĿ�ꡣ
�����ף�ͨ������������Ӧ���ɵ����������ʵ��̽��ľĿ�ꡣ ��2��ѡ��B�� ����������ţ�����װ�ý���ʵ�顣
��2��ѡ��B�� ����������ţ�����װ�ý���ʵ�顣 ��3����ó�ַ�Ӧ���������������ΪVL����״������
��3����ó�ַ�Ӧ���������������ΪVL����״������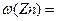 = ��
= �� ��4������Ʋ��ȣ�����Ҫ������һ���������� ��
��4������Ʋ��ȣ�����Ҫ������һ���������� �� ��5����װ��B�еĺ�ѹ��Һ©����Ϊ��ͨ��Һ©����������������ƫ����ƫС������Ӱ�족����
��5����װ��B�еĺ�ѹ��Һ©����Ϊ��ͨ��Һ©����������������ƫ����ƫС������Ӱ�족���� �����ң�ͨ������������Ӧǰ�������ʵ��̽��Ŀ�ꡣѡ������C��ʵ�飬��������ַ�Ӧ���˳������ϴ�ӡ���ɣ��Ƶ�������Ϊm2g ��
�����ң�ͨ������������Ӧǰ�������ʵ��̽��Ŀ�ꡣѡ������C��ʵ�飬��������ַ�Ӧ���˳������ϴ�ӡ���ɣ��Ƶ�������Ϊm2g �� ��6��
��6��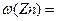 ��
�� ��������ͨ������������Ӧǰ���������������Լ��������������ֵ��ΪH2��������ʵ��̽��Ŀ�ꡣʵ��ͬ��ʹ������C��
��������ͨ������������Ӧǰ���������������Լ��������������ֵ��ΪH2��������ʵ��̽��Ŀ�ꡣʵ��ͬ��ʹ������C�� ��7����ʵ�����Ƕȷ����������� �����ң�����ڡ��������ڡ���ͬ�ڡ�����
��7����ʵ�����Ƕȷ����������� �����ң�����ڡ��������ڡ���ͬ�ڡ����� (1)C (2)D (3)
(1)C (2)D (3) 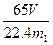 (��
(��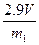 �����������𰸣�
�����������𰸣� ��4������п���ܶȣ������������𰸣�
��4������п���ܶȣ������������𰸣� ��5��ƫ��
��5��ƫ�� ��6��
��6��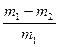 �������������𰸣�
�������������𰸣� ��7������
��7������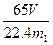 ����4������Zn��������������ܶȣ���������Zn�����������Zn�Ľ�������������Zn�ĸ߶ȣ���ȣ�����5����ѹʽ��Һ©�������������в��ֲ����ڷ�Һ©���Ϸ���������ʱ�ռ����ˣ���������ͨ©��ʱ�ռ���H2��һЩ����������Zn����ƫ��6�����ٵ�������ΪZn����������7������������H2��������ֵ����Ȼ������Ϊ������H2������С������ƫ���
����4������Zn��������������ܶȣ���������Zn�����������Zn�Ľ�������������Zn�ĸ߶ȣ���ȣ�����5����ѹʽ��Һ©�������������в��ֲ����ڷ�Һ©���Ϸ���������ʱ�ռ����ˣ���������ͨ©��ʱ�ռ���H2��һЩ����������Zn����ƫ��6�����ٵ�������ΪZn����������7������������H2��������ֵ����Ȼ������Ϊ������H2������С������ƫ���


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
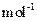 ��MnCO3 115 MnO2 87 MnO 71��
��MnCO3 115 MnO2 87 MnO 71���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ĵ�ͭ��������� |
| B�������ĵ�����������٣��ڣ��� |
| C���Ի�����ɵ�Σ��������С |
| D����ȡ����ͭ����ѷ����Ǣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
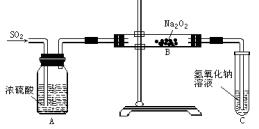
| ���� | �����Լ� | ������Լ���Ŀ�� |
| B | ����NaHCO3��Һ | ______ |
| C | ______ | ��ȥCO2�е�ˮ���� |
| E | ______ | ______ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ���� | ���� | �Լ� | ��Ҫ���� | |
| A | SiO2 | CaCO3 | ���� | ���� |
| B | NaHCO3 | Na2CO3 | ���� | ���� |
| C | Fe | Al | �������� | ��Һ |
| D | Fe��NO3��2 | Ba��NO3��2 | ���� | ���� |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| ʵ���Լ� | ʵ����� | ||
| �� | �� | �� | |
| �� | ������� | �� | �����ԣ�KMnO4��Cl2��Br2 |
| �Լ��� | �Լ��� | �Լ��� | �������� | ʵ����� |
| ______ | ______ | ______ | ______ | ______ |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com