
����12�֣�����ͼת����ϵ�У��������ɫ��Ӧ�ʻ�ɫ��MΪ������Һ�����ʣ�IΪ������������I��G��ϡ��Һ����Ӧ��ֻ����G��Ũ��Һ�ڼ��������·�Ӧ����G����Ҫ�Ļ�����Ʒ�ͻ���ԭ�ϣ�HΪ���õ��ͻ���ϣ�ͼ�в��ֲ���û���г�����
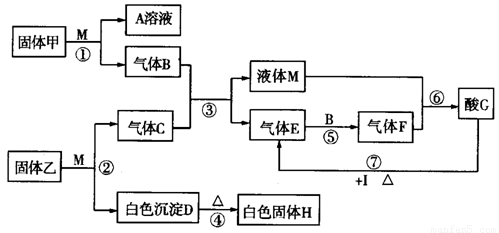 ��1�����������Ϊ__________��F�Ļ�ѧʽΪ______________��
��1�����������Ϊ__________��F�Ļ�ѧʽΪ______________��
��2����Ӧ�١���������������ԭ��Ӧ����______________����д��Ӧ��ţ���
��3����ҵ�����г���������E��Ϊ�˳��������Դ������������ĿǰӦ����㷺�Ĺ�ҵ��������������ʯ�ҷ�������ʯ��Ϊԭ��������װ����������E��Ӧ�Ļ�ѧ����ʽΪ��
___________________________________________��
��4����Ӧ�ߵĻ�ѧ��Ӧ����ʽΪ_____________________________________________��
��5����֪��ҵ�ϵ�����ڵ�Hұ����������X����40��0g���������У�����H��Fe2O3��SiO2������������ϡ���ᣬ����õ�12��0g���壻��Һ�м������A��Һ������õ�21��4g���壻��˻������Ԫ��X����������Ϊ__________________��
 ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| �� |
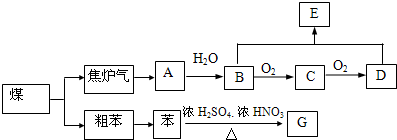




| ϡ���� |
| �� |
| ϡ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼת����ϵ�У��������ɫ��Ӧ�ʻ�ɫ��MΪ������Һ�����ʣ���G����Ҫ�Ļ�����Ʒ�ͻ���ԭ�ϣ�����H���ܽ���A��Һ����G�У���HΪ���õ��ͻ����(ͼ�в��ֲ���û���г�)��

��1��д��M�����Ĺ����Ӧ�Ļ�ѧ����ʽ��
��2����ͼ��ʾ����������G�Ĺ�ҵ���̣�

���豸�ҵ�����Ϊ ��
������X����Ҫ�ɷ�Ϊ ��
��д���豸���г����Ļ�ѧ��Ӧ ��
��3����������M��Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ������ͨ���и����ڶ������Ͽ��ԣ����ۣ���ѧ���� ���ͣ������
��15�֣�I����ͼת����ϵ�У��������ɫ��Ӧ�ʻ�ɫ��MΪ������Һ�����ʣ���G����Ҫ�Ļ�����Ʒ�ͻ���ԭ�ϣ�����H���ܽ���A����G��HΪ���õ��ͻ����(ͼ�в��ֲ���û���г�)��
II��ͼ��ʾ����������G�Ĺ�ҵ���̣�
��1�����豸������Ϊ �����ҵĻ�ѧʽΪ M���ӵĽṹʽΪ ��
��2������X����Ҫ�ɷ�Ϊ ��
��3��д���豸���г����Ļ�ѧ��Ӧ ��
��4����ɫ����D��G��Һ��Ӧ�����ӷ���ʽΪ ��
��5����2.24 L����״���£�Eͨ��100 mL2 mol/L A��ˮ�� Һ����Һ������Ũ���ɴ�С��˳��Ϊ____________ ___��
Һ����Һ������Ũ���ɴ�С��˳��Ϊ____________ ___��
��6��������ڹ���H���Ƶ�һ�ֽ��������⻯ѧ����ʽΪ �����ʱת��2.4mol���ӣ��Ƶý��� g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡ�����и����ڶ���ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ������
����ͼת����ϵ�У��������ɫ��Ӧ�ʻ�ɫ��MΪ������Һ�����ʣ�IΪһ������������G����Ҫ�Ļ�����Ʒ�ͻ���ԭ�ϣ�����H�ܹ��ܽ���A��Һ����G�У���HΪ���õ��ͻ���ϣ�ͼ�в��ֲ���û���г�����

��1���������Һ��M��Ӧ�ķ���ʽΪ______��A��Һ�����H��Ӧ�����ӷ���ʽΪ______��
��2�������ҵĻ�ѧʽΪ_______��Һ��M�ĵ���ʽΪ_______��
��3����Ӧ������������������ԭ��Ӧ��Ϊ_______����д��Ӧ��ţ���
��4����I��C��ϡ��Һ����Ӧ��ֻ����G��Ũ��Һ�ڼ��������·�Ӧ����Ӧ���Ļ�ѧ����ʽΪ_______��
��5�����ɻ�����(FeS2)������B��Ӧ����������E����ÿ����1 mol E�ų�426.5 kJ���������÷�Ӧ���Ȼ�ѧ����ʽΪ_______ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com