
| �������� | ��ʼ������pH | ��ȫ������pH |
| Fe3+ | 1.1 | 2.8 |
| A13+ | 3.4 | 4.7 |
| Fe2+ | 5.8 | 8.8 |
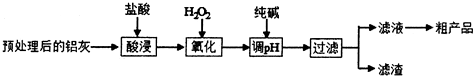
���� ��1������������лӷ��Ժ��¶ȶԷ�Ӧ���ʵ�Ӱ��Ƕȷ�����
��2��ʵ��Ŀ�����Ʊ�������[Al��OH��2Cl]������Ҫ��ȥ�����Ӻ��������ӣ���������������������pH�ϸߣ���Ҫ��˫��ˮ������������ȫת���������ӣ��ݴ�д����Ӧ�����ӷ���ʽ��
��3�����ݱ�����������ȫ�����������ӿ�ʼ������pH���ݷ���������Һ��pH��Χ��
��4��������ҺpH����������ȫת�������������������ٽ�϶������費�����ᷴӦ��֪�����ijɷ֣�
��� �⣺��1�����¶�̫�ͣ���Ӧ�����¶�̫�ߣ�����ӷ��죬�����ܡ��¶ȿ�����30�桫35�棬����̫�ͣ�Ҳ����̫�ߣ�
�ʴ�Ϊ���¶�̫�ͣ���Ӧ�����¶�̫�ߣ�����ӷ��죻
��2���Ʊ�������[Al��OH��2Cl]������Ҫ��ȥ�����Ӻ��������ӣ���������������������pH�ϸߣ����������ӣ�����Ҫ��˫��ˮ������������ȫת���������ӣ���Ӧ�����ӷ���ʽΪ��2Fe2++H2O2+2H+=2Fe3++2H2O��
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��3�����ݱ������ݿ�֪��pH=2.8ʱFe3+��ȫ������pH=3.4ʱA13+��ʼ������Ҫ��ȥ��Һ�е������Ӷ�����ʹ�����ӳ�����Ҫ����Һ��pH��ΧΪ��2.8��pH��3.4��
�ʴ�Ϊ��2.8��pH��3.4��
��4���ô��������ҺpH����������ȫת����Fe��OH��3�����������к���Fe��OH��3������SiO2�������ᷴӦ������������Ҫ�ɷ�ΪSiO2��Fe��OH��3��
�ʴ�Ϊ��SiO2��Fe��OH��3��
���� ���⿼�����Ʊ���������ƣ���Ŀ�Ѷ��еȣ������Ʊ���������ʵ��Ŀ�ġ�ʵ��ԭ��Ϊ���ؼ�����2��Ϊ�״��㣬ע���������������ʼ����������������ȫ������pH����������������ѧ���ķ�����������ѧʵ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и����Ͼ���������ѧ�Ծ��������棩 ���ͣ�ѡ����
������Ԫ��W��X��Y��Z��ԭ��������������W��Y����������֮��ΪX��������������2 ����Z�����������������ڲ��������X��Y��Z�ļ����ӵĵ��Ӳ�ṹ��ͬ��W�ĵ����ǿ�������������������塣����˵����ȷ���ǣ� ��
A. Y������������Ӧˮ��������Ա�W��ǿ
B. W����̬�⻯���X���ȶ�
C. ���Ӱ뾶�Ĵ�С˳��r(W)��r(X)��r(Y)��r(Z)
D. XY2��ZY2�еĻ�ѧ��������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�츣��ʡ�����ϵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��Na2O2?Na2CO3?NaHCO3?NaCl�е�ij������ɵĻ����,�����м�������������������ų�?������ͨ��������NaOH��Һ,�����������һ����?������������ڿ����м���,������ų�,�����ж���ȷ����( )
A���������һ������Na2CO3?NaCl B���������һ����Na2O2?NaHCO3
C����ȷ����������Ƿ���NaHCO3 D���������һ������Na2O2?NaCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ������ȣ���ȵ�λ��g/100ml�����ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ���ұ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��
ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ������ȣ���ȵ�λ��g/100ml�����ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ���ұ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��| ָʾ�� | ʯ�� | ���� | ���� | ��̪ |
| ��ɫ��Χ ��PH�� | 5.0-8.0 | 3.1-4.4 | 4.4-6.2 | 8.2-10.0 |
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/ml | 26.02 | 25.32 | 25.28 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ����/��mol��L-1�� | Pb2+ | Ca2+ | Fe3+ | Mn2+ | Cl- |
| ����ǰŨ�� | 0.100 | 29.8 | 0.120 | 0.087 | 51.9 |
| ������Ũ�� | 0.004 | 22.6 | 0.040 | 0.053 | 49.9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�������и�һ��10���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
ʵ���������ô�����KCl���壬��Ŀǰֻ�к�K2SO4����NH4��2CO3���ʵ�KCl��ijѧ���������ͼ��ʾ���������ᴿ��
��֪��1����NH4��2CO3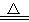 2NH3��+CO2��+H2O
2NH3��+CO2��+H2O
2��K2SO4��KCl���ȶ��Ժã�KCl�ܽ�����¶ȱ仯����
����������Ϣ���ش��������⣺
��1������ټ��ȵ�Ŀ���ǣ�______________���˲�����ѡ����______________�����������ƣ��н��У�
��2������ڲ������ᱵ��Һ����������_________________��
��3�����в����ʱ�������ж�SO42-�Ƿ������ʵ�����������ͽ��ۣ�____________��
��4������������Լ�MΪ��_______________����Ŀ����_________________��
��5������S��������__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 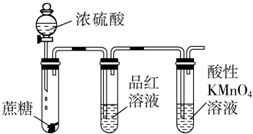 ����Ũ���������Ƿ�Ӧ�����Ķ������� | |
| B�� | 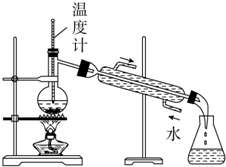 ����ױ���ˮ | |
| C�� | 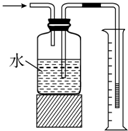 ������������� | |
| D�� | 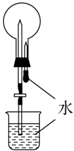 ���ж�����̼��Ȫʵ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com