
(14 �֣�
����Ҫ��������и�С��ʵ��Ŀ�ġ���a��bΪ���ɼУ����ȼ��̶�װ������ȥ��
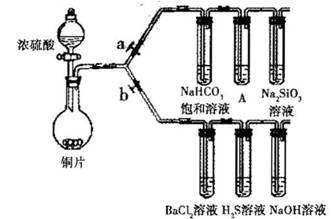
(1)��֤̼����ǽ����Ե����ǿ��������֪����:������ >̼�ᣩ
����������. ________����ҩƷ��a�ر� b,Ȼ����������,����
��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ��________________װ��A�����Լ���________
����˵��̼�ķǽ����Աȹ�ǿ��ʵ�������ǣ�________
(2)��֤ SO,��������.����ԭ�Ժ������������ͨ�ԡ�
�ٴ�b���ر�a��
��H2S��Һ���dz��ɫ���dz���,��ѧ����ʽ�ǣ�_____
��BaCl2��Һ����߶��������ֳ����ݣ��քe�μ�������Һ���������ij����Ļ�ѧʽ�����±���Ӧλ��
|
�μӵ���Һ |
��ˮ |
��ˮ |
|
�����Ļ�ѧʽ |
|
|
д������SO2 ��ʾ��ԭ�Բ����ɳ��������ӷ���ʽ_________
 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д� һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
( 14��)Ϊ��̽��Ũ�ȶ����������Ե�Ӱ�죬ijѧϰС�����������̽�����
[̽��һ]
��ȡ����������̼�ظ֣�12.0g����30.0mLŨ�����У����ȣ���ַ�Ӧ��õ���ҺX���ռ�������Y��
��1����ͬѧ��ΪX�г�Fe3+����ܺ���Fe2+����Ҫȷ�����е�Fe2+����ѡ�������Լ�����Ƽ�ʵ�鷽���������������̡�����ͽ��ۣ� ��
����ѡ���Լ���.KSCN��Һ����ˮ�����ۣ�.Ũ��ˮ ������KMnO4��Һ����
��2����ͬѧΪ�˲ⶨ����Y��SO2�ĺ���������������ַ�����
����I��ȡ672mL����״��������Yͨ��������ˮ�У�Ȼ���������BaCl2��Һ�����ʵ�������ø������4.66g��
����II����VmL c mol��L-1���Ը��������Һ�л���ͨ��Y����aL����״��������Һǡ����ȫ��ɫ��
����III��ȡVL����״��������Y����ͨ������������������Һ�У���ַ�Ӧ���ˡ�ϴ�ӡ���ɣ��Ƶù�������Ϊmg��
���в������ķ����� �������� ��
[̽����]
��������ʵ����SO2��������Ľ������ͬѧ��Ϊ����Y�л����ܺ�����H2��CO2
���塣Ϊ�����������̽��ʵ��װ�ã�ͼ�мг�װ����ʡ�ԣ���
��3��װ��A���Լ��������� ��
��4������ȷ������Y�к���CO2��ʵ������ ��
��5����ͬѧ���ݡ�F�������ˮ����ͭ�Ƿ����ɫ��ȷ��Y�������Ƿ�������������Ϊ�Ƿ�ɿ��� ����ɿ������ɿ��������������ɣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(14��)ij�Ȼ�����Ʒ��������FeCl2���ʡ���Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�
��������������̣��ش��������⣺
(1)���� I ���õ��IJ����������ձ�������������Ͳ�⣬�������� (����ѡ��������)������ II �����õ��������� (������ѡ����ѡ������)��
��A��50mL�ձ�������B��50mL��Ͳ C��25mL��ʽ�ζ��ܡ���D��25mL��ʽ�ζ���
(2)�����Լ��ٺ͢ں�����Ӧ�����ӷ�Ӧ����ʽΪ��
(3) ����������ȣ������ڸ���������ȴ�����£�����ƽ����������Ϊb1g���ٴμ��Ȳ���ȴ�����³���������Ϊb2 g����b1��b2=0.3 g�����������Ӧ���еIJ�����____________��
(4) ������������W1g����������Ⱥ������������W2g������Ʒ����Ԫ�ص����������ǣ� ��
(5) ��ͬѧ��Ϊ����������������������ˮ���������費�䣬�ԿɴﵽĿ�ġ����������ǣ�
(�û�ѧ����ʽ��ʾ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ĵ�ʡ����9���¿���ѧ���� ���ͣ�ʵ����
�� 14 �֣�ij��ɫ����Һ���ܺ����������ӣ�K+��Fe3+��Cu2+��Ba2+��AlO��NO3����CO32����SO32����SO42����Br����I����Cl����ȡ����Һ�����²������ʵ�顣
��1����������±������е�ʵ�������ش�������й����⣺
|
��ʵ���� |
��ش����� |
|
��ȡԭ��Һ�������μ�������ˮ���������������ˣ���Һ��CCl4����CCl4��δ��ɫ�� |
�ٸò�ʵ��˵����Һ�в����� ����_________________�� |
|
��ȡԭ��Һ����������ͭƬ��ϡ���Ṳ�ȣ�������ɫ���壬������������������Ϊ����ɫ�� |
�ڸò�ʵ�鷢����Ӧ�����ӷ���ʽΪ _________________�� |
|
��ȡԭ��Һ�������������ữ���백ˮ�а�ɫ�������ɣ��������������ˮ����������ʧ�� |
�۲�����ɫ���������ӷ���ʽΪ _________________�� |
|
��ȡԭ��Һ�������μ�2�����Ը��������Һ����ɫ������ȥ�� |
�ܸò�ʵ��˵����Һ�д��� ���� _________________�� |
|
��ȡԭ��Һ������������Ba��NO3��2��Һ������ɫ���������ˣ���ϴ�Ӻ�ij����м����������ᣬ���������ܽ⣬����ɫ��������� |
��δ�ܽ�İ�ɫ������ ��������ͨ��Ʒ����Һ�������� �� |
|
��ȡ�����������Һ����HNO3�ữ���ټ�AgNO3��Һ����Һ��������ɫ������ |
�ò����Ƿ������� ����С����ޡ����� |
��2�����ϣ�����Һ��pH ���������������������7������Һ��һ�����ڵ��������� ������ȷ���Ƿ���ڵ������� ������ȡԭ��Һ�������Ƿ���ڣ��Ƿ�Ҫʹ���Լ�������Ҫʹ���Լ�����ʵ�鷽���� ����Ҫʹ���Լ���ѡ�õ��Լ��ǣ���ʹ�õ��Ⱥ�˳������д����
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
����14�֣��±���Ԫ�����ڱ��е�һ���֣�����A��I�����ڱ��е�λ�ã���Ԫ�ط��Ż�ѧʽ�ش��������⣺
|
|
��A |
|
|
|
|
|
|
0 |
|
1 |
|
��A |
��A |
��A |
��A |
��A |
��A |
|
|
2 |
|
|
|
D |
E |
|
G |
I |
|
3 |
A |
B |
C |
|
F |
|
H |
|
��1������Ԫ�أ���ѧ��������õ��� ����������ǿ�ĵ����� ����ԭ����ǿ�ĵ����� ��
��2������������ˮ���������ǿ���� ��������ǿ���� �������Ե��� ��
��3��Ҫ֤��A��B��C�Ľ��������ԣ�������ʲôʵ����֤�����Ծ�һ��
ʵ����� ��
ʵ������ ��
�йػ�ѧ����ʽ��
(4)G��H����̬�⻯���ȶ��� > ��˵����Ӧ���ʵķǽ����� > ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10��̨���и߶���ѧ����ĩ���Ի�ѧ�� ���ͣ������
(14��) ���ڷ�ӦA(g) 2B(g) DH>0�����¶�ΪT1��T2ʱ��ƽ����ϵ��B�����������ѹǿ�仯��������ͼ��ʾ���ش����и��⡣

��1����������ͼ����������˵����ȷ���� �� (����ĸ) ��
A��a��c����ķ�Ӧ���ʣ�a ��c
B����״̬b��״̬a������ͨ�����ȵķ���
C��b��c����A�����ת�������
��2��������Ӧ���ܱ����������ݣ��н��У��ﵽƽ��״̬�ı�־�� �� (����ĸ) ��
A����λʱ��������n mol A��ͬʱ�ֽ�2n molB
B���������������������ٸı�
C��v����A����2v����B��
D�����������ܶȲ��ٷ����仯
E����������ѹǿ���ٷ����仯
��3����������Ӧ��ƽ��ʱ��B�����ƽ��Ũ��Ϊ0.1 mol��L-1 ��ͨ����С�����������ϵ��ѹǿ(�¶ȱ��ֲ���)�����´�ƽ���B�����ƽ��Ũ�� �� 0.1 mol��L-1�����������������=���� ��
��4����100��ʱ����0.40mol��B�������2L��յ��ܱ������У�ÿ��һ��ʱ��ͶԸ������ڵ����ʽ��з������õ����±������ݣ�
|
ʱ�䣨s�� |
0 |
20 |
40 |
60 |
80 |
|
n(B)/mol |
0.40 |
n1 |
0.26 |
n3 |
n4 |
|
n(A)/mol |
0.00 |
0.05 |
n2 |
0.08 |
0.08 |
�� �����������£��ӷ�Ӧ��ʼ��40sʱ����A�����ʾ�ĸ÷�Ӧ��ƽ����Ӧ����Ϊ �� ��
�� �ϱ���n3 �� n4�����������������=��������ӦA(g) 2B(g)��100��ʱ��ƽ�ⳣ��K��ֵΪ �� �������¶Ⱥ�Ӧ2B(g) A(g)��ƽ�ⳣ��K��ֵ �� �����������С�����䡱����
�� ������ͬ����������������г������A���壬Ҫ�ﵽ����ͬ����ƽ��״̬��A�������ʼŨ��Ϊ �� mol��L-1 ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com