
”¾ĢāÄæ”æĮ×Ėį¶žĒā¼Ų£ØKH2PO4£©ŹĒŅ»ÖÖøߊ§ø“ŗĻ·Ź”£¹¤ŅµÉĻŅŌĮ×¾«æó[Ö÷ŅŖ³É·ÖŹĒCa3(PO4)2£¬»¹ŗ¬ÓŠÉŁĮæFe2O3”¢CaF2µČŌÓÖŹ]ĪŖŌĮĻ£¬Éś²śĮ×Ėį¶žĒā¼ŲµÄĮ÷³ĢČēĶ¼£ŗ

ŅŃÖŖ£ŗ¢ŁTBP”¢D2EHPA”¢TOA¶¼ŹĒÄŃČÜÓŚĖ®µÄŅŗĢ¬ÓŠ»śĪļ£¬¶ŌŻĶČ”ĢŲ¶ØĪļÖŹÓŠ½ĻĒæµÄŃ”ŌńŠŌ£¬³£ÓĆ×÷ŻĶČ”¼Į”£
¢ŚŻĶČ”¼ĮTBP¶ŌH3PO4ŗĶFe3+ÓŠ½ĻĒæµÄŻĶČ”×÷ÓĆ£¬µ«¶ŌCa2+ÓŠŅ»¶ØµÄŻĶČ”×÷ÓĆ”£
¢ŪŻĶČ”¼ĮD2EHPA½ö¶ŌFe3+ÓŠ½ĻĒæµÄŻĶČ”×÷ÓĆ”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©”°ÖĘĖį”±¹ż³ĢÖŠÉś³ÉĮ×ĖįµÄ»Æѧ·½³ĢŹ½ĪŖ______”£
£Ø2£©”°³ż·ś”±Ź±·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ______”£
£Ø3£©”°ÄæµÄ1”±ŹĒ______”£
£Ø4£©”°ÄæµÄ2”±·ÖĄė³öµÄÓŠ»ś²ćÖŠ³żH3PO4Ķā£¬»¹ÓŠÉŁĮæij½šŹōŃōĄė×Ó”£Č„³żÓŠ»ś²ćÖŠøĆŃōĄė×ӵķ½·ØŹĒÓĆŗ¬H2SO4µÄĮ×ĖįĻ“µÓ£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ______”£
£Ø5£©”°·“Ó¦”±Ź±ĻČŌŚĮ×ĖįÖŠ¼ÓČėKCl£¬ŌŁ¼ÓČėTOA£¬TOAµÄ×÷ÓĆŹĒ______”£
£Ø6£©”°·“Ó¦”±ÖŠ£¬TOAµÄÓĆĮæ»įÓ°ĻģČÜŅŗµÄpH”£Ė®ČÜŅŗÖŠH3PO4”¢H2PO4-”¢HPO42-”¢PO43-µÄ·Ö²¼·ÖŹż¦Ä£Øŗ¬Į×ŌŖĖŲĪ¢Į£Õ¼Č«²æŗ¬Į×Į£×ÓµÄĪļÖŹµÄĮæ·ÖŹż£©ĖępHµÄ±ä»ÆČēĶ¼ĖłŹ¾”£

”°·“Ó¦”±ÖŠ£¬µ±pH=______£ØĢīŃ””°2.2”±”¢”°4.5”±”¢”°9.5”±»ņ”°12.4”±£©Ź±£¬Ķ£Ö¹¼ÓČėTOA”£
”¾“š°ø”æCa3(PO4)2+6HCl£½3CaCl2+2H3PO4 SiO2+4HF£½SiF4”ü+2H2O ³żČ„Į×ĖįÖŠŗ¬ÓŠµÄFe3+ ![]() Ė®ĻąÖŠ“ęŌŚKCl+H3PO4
Ė®ĻąÖŠ“ęŌŚKCl+H3PO4![]() HCl+KH2PO4£¬¼ÓČėTOA½«HCl×ŖŅʵ½ÓŠ»ś²ć£¬Ę½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶Æ£¬ÓŠĄūÓŚKH2PO4Éś³É 4.5
HCl+KH2PO4£¬¼ÓČėTOA½«HCl×ŖŅʵ½ÓŠ»ś²ć£¬Ę½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶Æ£¬ÓŠĄūÓŚKH2PO4Éś³É 4.5
”¾½āĪö”æ
ŅŌĮ×¾«æó[Ö÷ŅŖ³É·ÖŹĒCa3(PO4)2£¬»¹ŗ¬ÓŠÉŁĮæFe2O3”¢CaF2µČŌÓÖŹ]ĪŖŌĮĻ£¬Éś²śĮ×Ėį¶žĒā¼Ų£¬ÓÉĮ÷³ĢæÉÖŖ£¬¼ÓŃĪĖį·¢ÉśCa3(PO4)2+6HCl£½3CaCl2+2H3PO4”¢Fe2O3+6HCl£½2FeCl3+3H2O”¢CaF2+2HCl£½CaCl2+2HF£¬¼ÓČė»īŠŌ¶žŃõ»Æ¹č·¢ÉśSiO2+4HF=SiF4”ü+2H2O£¬D2EHPA½ö¶ŌFe3+ÓŠ½ĻĒæµÄŻĶČ”×÷ÓĆæɳżČ„Į×ĖįÖŠŗ¬ÓŠµÄFe3+£¬·ÖŅŗČ”Ė®²ć¼ÓTBPŻĶČ”H3PO4£¬·ÖŅŗȔӊ»ś²ćÕōĮó·ÖĄė³öĮ×Ėį£¬¼ÓČėKClÓėĻ”Į×ĖįÉś³ÉKH2PO4£¬ŌŁ¼ÓČėÓŠ»ś¼ī-ČżŠĮ°·(TOA)·ÖĄė£¬¶ŌĖ®²ć½į¾§æɵĆKH2PO4²śĘ·£¬ŅŌ“ĖĄ“½ā“š”£
£Ø1£©”°ÖĘĖį”±¹ż³ĢÖŠÉś³ÉĮ×ĖįµÄ»Æѧ·½³ĢŹ½ĪŖCa3(PO4)2+6HCl£½3CaCl2+2H3PO4£»
£Ø2£©HFÄÜÓė¶žŃõ»Æ¹č·“Ó¦£¬Ōņ”°³ż·ś”±Ź±·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖSiO2+4HF£½SiF4”ü+2H2O£»
£Ø3£©ÓÉÓŚŻĶČ”¼ĮD2EHPA½ö¶ŌFe3+ÓŠ½ĻĒæµÄŻĶČ”×÷ÓĆ£¬Ņņ“Ė”°ÄæµÄ1”±ŹĒ³żČ„Į×ĖįÖŠŗ¬ÓŠµÄFe3+£»
£Ø4£©”°ÄæµÄ2”±·ÖĄė³öµÄÓŠ»ś²ćÖŠ³żH3PO4Ķā£¬»¹ÓŠÉŁĮæij½šŹōŃōĄė×ÓøĘĄė×Ó”£Č„³żÓŠ»ś²ćÖŠøĆŃōĄė×ӵķ½·ØŹĒÓĆŗ¬H2SO4µÄĮ×ĖįĻ“µÓ£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ![]() £»
£»
£Ø5£©”°·“Ó¦”±Ź±ĻČŌŚĮ×ĖįÖŠ¼ÓČėKCl£¬ŌŁ¼ÓČėTOA£¬ÓÉÓŚĖ®ĻąÖŠ“ęŌŚKCl+H3PO4![]() HCl+KH2PO4£¬¼ÓČėTOA½«HCl×ŖŅʵ½ÓŠ»ś²ć£¬Ę½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶Æ£¬ÓŠĄūÓŚKH2PO4Éś³É£»
HCl+KH2PO4£¬¼ÓČėTOA½«HCl×ŖŅʵ½ÓŠ»ś²ć£¬Ę½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶Æ£¬ÓŠĄūÓŚKH2PO4Éś³É£»
£Ø6£©ÓÉĶ¼æÉÖŖ£¬BĪŖ²śĪļ![]() µÄĮ棬Ėę×ÅpHµÄŌö“ó£¬
µÄĮ棬Ėę×ÅpHµÄŌö“ó£¬![]() µÄĮæŌö“󣬵±pH=4.5Ź±£¬
µÄĮæŌö“󣬵±pH=4.5Ź±£¬![]() ×ī¶ą£¬pHŌŁÉśøߣ¬²»ĄūÓŚ
×ī¶ą£¬pHŌŁÉśøߣ¬²»ĄūÓŚ![]() µÄÉś³É£¬¹ŹpH=4.5£¬Ķ£Ö¹¼ÓČėTOA”£
µÄÉś³É£¬¹ŹpH=4.5£¬Ķ£Ö¹¼ÓČėTOA”£


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æAŹĒŅ»ÖÖŠÅĻ¢²ÄĮĻµÄĢķ¼Ó¼Į£¬ŌŚĻąĶ¬Ģõ¼žĻĀ£¬AÕōĘųŹĒĶ¬Ģå»żĒāĘųÖŹĮæµÄ88.25±¶”£ŌŚA·Ö×ÓÖŠø÷ŌŖĖŲÖŹĮæ·ÖŹż·Ö±šĪŖw(C)£½54.4%£¬w(H)£½7.4%£¬w(O)£½18.1%£¬w(Cl)£½20.1%£¬AŌŚ²»Ķ¬Ģõ¼žĻĀæÉ·¢ÉśČēĶ¼ĖłŹ¾µÄŅ»ĻµĮŠ±ä»Æ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)AµÄ·Ö×ÓŹ½ĪŖ__________”£
(2)D·Ö×Óŗ¬ÓŠµÄ¹ŁÄÜĶÅŹĒ________________”£
(3)ÉĻŹö×Ŗ»»¹ŲĻµµÄ»Æѧ·½³ĢŹ½ÖŠ£¬ŹōÓŚĖ®½ā·“Ó¦µÄÓŠ________øö(ĢīŹż×Ö)”£
(4)Š“³ö»Æѧ·½³ĢŹ½£ŗ
¢ŁAŗĶĻ”ĮņĖį¹²ČČ£ŗ____________________________________________________£»
¢ŚEŃõ»Æ³ÉG£ŗ__________________________________________________£»
¢ŪFÓėĒāŃõ»ÆÄĘ“¼ČÜŅŗ¹²ČČ£ŗ__________________________________________”£
(5)ÓėB»„ĪŖĶ¬·ÖŅģ¹¹ĢåµÄĒŅŹōÓŚĮ“דõ„µÄĪļÖŹ¹²ÓŠ____ÖÖ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ·Ö×Ó»ņĄė×ÓÖŠ¼ü½ĒÓɓ󵽊”ÅÅĮŠµÄŹĒ£Ø £©
¢ŁBCl3 ¢ŚNH3 ¢ŪH2O ¢ÜPCl4+ ¢ŻHgCl2
A.¢Ż¢Ü¢Ł¢Ś¢ŪB.¢Ż¢Ł¢Ü¢Ś¢ŪC.¢Ü¢Ł¢Ś¢Ż¢ŪD.¢Ū¢Ś¢Ü¢Ł¢Ż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÅšĖį(H3BO3)ŹĒŅ»ÖÖʬ²ćד½į¹¹°×É«¾§Ģ壬ŹÜČČŅ×·Ö½ā”£²ćÄŚµÄ H3BO3·Ö×ÓĶعżĒā¼üĻąĮ¬(ČēĶ¼ĖłŹ¾)£¬ŌņĻĀĮŠÓŠ¹ŲĖµ·ØÖŠÕżČ·µÄŹĒ

A.ÕżÅšĖį¾§ĢåŹōÓŚŌ×Ó¾§ĢåB.H3BO3·Ö×ÓµÄĪČ¶ØŠŌÓėĒā¼üÓŠ¹Ų
C.1 mol H3BO3¾§ĢåÖŠŗ¬ÓŠ3 molĒā¼üD.·Ö×ÓÖŠÅšŌ×Ó×īĶā²ćĪŖ8µē×ÓĪČ¶Ø½į¹¹
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æAŹĒŅ»ÖÖŠÅĻ¢²ÄĮĻµÄĢķ¼Ó¼Į£¬ŌŚĻąĶ¬Ģõ¼žĻĀ£¬AÕōĘųŹĒĶ¬Ģå»żĒāĘųÖŹĮæµÄ88.25±¶”£ŌŚA·Ö×ÓÖŠø÷ŌŖĖŲÖŹĮæ·ÖŹż·Ö±šĪŖw(C)£½54.4%£¬w(H)£½7.4%£¬w(O)£½18.1%£¬w(Cl)£½20.1%£¬AŌŚ²»Ķ¬Ģõ¼žĻĀæÉ·¢ÉśČēĶ¼ĖłŹ¾µÄŅ»ĻµĮŠ±ä»Æ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)AµÄ·Ö×ÓŹ½ĪŖ__________”£
(2)D·Ö×Óŗ¬ÓŠµÄ¹ŁÄÜĶÅŹĒ________________”£
(3)ÉĻŹö×Ŗ»»¹ŲĻµµÄ»Æѧ·½³ĢŹ½ÖŠ£¬ŹōÓŚĖ®½ā·“Ó¦µÄÓŠ________øö(ĢīŹż×Ö)”£
(4)Š“³ö»Æѧ·½³ĢŹ½£ŗ
¢ŁAŗĶĻ”ĮņĖį¹²ČČ£ŗ____________________________________________________£»
¢ŚEŃõ»Æ³ÉG£ŗ__________________________________________________£»
¢ŪFÓėĒāŃõ»ÆÄĘ“¼ČÜŅŗ¹²ČČ£ŗ__________________________________________”£
(5)ÓėB»„ĪŖĶ¬·ÖŅģ¹¹ĢåµÄĒŅŹōÓŚĮ“דõ„µÄĪļÖŹ¹²ÓŠ____ÖÖ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æijӊ»śĪļA(C4H6O5)¹ć·ŗ“ęŌŚÓŚŠķ¶ąĖ®¹ūÄŚ£¬ÓČŅŌĘ»¹ū”¢ĘĻĢŃ”¢Ī÷¹Ļ”¢É½é«ÄŚĪŖ¶ą£¬ŹĒŅ»ÖÖ³£ÓƵď³Ę·Ģķ¼Ó¼Į”£øĆ»ÆŗĻĪļ¾ßÓŠČēĻĀŠŌÖŹ£ŗ
(i)ŌŚ25”ꏱ£¬µēĄėĘ½ŗā³£ŹżK£½3.9”Į10-4£¬K2£½5.5”Į10-6
(ii)A+RCOOH(»ņROH)![]()
![]() ÓŠĻćĪ¶µÄ²śĪļ
ÓŠĻćĪ¶µÄ²śĪļ
(iii)1molA![]() ĀżĀż²śÉś1.5molĘųĢå
ĀżĀż²śÉś1.5molĘųĢå
(iv)ŗĖ“Ź²ÕńĒāĘ×ĖµĆ÷A·Ö×ÓÖŠÓŠ5ÖÖ²»Ķ¬»Æѧ»·¾³µÄĒāŌ×ÓÓėAĻą¹ŲµÄ·“Ó¦æņĶ¼ČēĻĀ£ŗ

£Ø1£©ŅĄÕÕ»ÆŗĻĪļAµÄŠŌÖŹ£¬¶ŌAµÄ½į¹¹æÉ×÷³öµÄÅŠ¶ĻŹĒ___”£
a£®Č·ŠÅÓŠĢ¼Ģ¼Ė«¼ü b£®ÓŠĮ½øöōČ»ł c£®Č·ŠÅÓŠōĒ»ł d£®ÓŠ£COOR¹ŁÄÜĶÅ
£Ø2£©Š“³öA”¢FµÄ½į¹¹¼ņŹ½£ŗA£ŗ__”¢F£ŗ__”£
£Ø3£©Š“³öA”śB”¢B”śEµÄ·“Ó¦ĄąŠĶ£ŗA”śB___”¢B”śE__”£
£Ø4£©Š“³öŅŌĻĀ·“Ó¦µÄ·“Ó¦Ģõ¼ž£ŗE”śFµŚ¢Ł²½·“Ó¦__”£
£Ø5£©ŌŚ“߻ƼĮ×÷ÓĆĻĀ£¬BÓėŅŅ¶ž“¼æÉ·¢ÉśĖõ¾Ū·“Ó¦£¬Éś³ÉµÄøß·Ö×Ó»ÆŗĻĪļÓĆÓŚÖĘŌģ²£Į§øÖ”£Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ__”£
£Ø6£©Š“³öÓėA¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄAµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£ŗ___”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĮņĖįŹĒÓĆĶ¾¹ć·ŗµÄ»Æ¹¤ŌĮĻ£¬æÉ×÷ĶŃĖ®¼Į”¢ĪüĖ®¼Į”¢Ńõ»Æ¼ĮŗĶ“߻ƼĮµČ”£
£Ø1£©¹¤ŅµÖĘĮņĖįĶµÄ·½·Øŗܶą”£
¢Ł ·½·ØŅ»”¢ÓĆÅØĮņĖįŗĶĶÖĘČ”ĮņĖįĶ”£øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ________£¬“Ė·ØµÄ×ī“óȱµćŹĒ__________”£
¢Ś·½·Ø¶ž”¢ÓĆĻ”ĮņĖį”¢ĶŗĶŃõ»ÆĢśÖĘČ”ĮņĖįĶ£¬Éś²śµÄÖ÷ŅŖ¹ż³ĢČēĻĀĶ¼ĖłŹ¾£ŗ

Ļ”ĮņĖį”¢ĶŗĶŃõ»ÆĢś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_________________£»Ļņ»ģŗĻČÜŅŗÖŠĶØČėČČæÕĘųµÄ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ___________________£»ÓÉĀĖŅŗµĆµ½ĪŽĖ®ĮņĖįĶµÄŹµŃé²Ł×÷ŹĒ______________”£
£Ø2£©°±·ØĶŃĮņ¼¼ŹõæÉĪüŹÕĮņĖį¹¤ŅµĪ²ĘųÖŠµÄ¶žŃõ»ÆĮņ£¬Ķ¬Ź±ÖʵĆĮņĖįļ§”£Ö÷ŅŖµÄ¹¤ŅÕĮ÷³ĢČēĻĀĶ¼ĖłŹ¾£ŗ

¢ŁĪüŹÕĖžÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_______________________”£
¢Ś ÓŠŹż¾Ż±ķĆ÷£¬ĪüŹÕĖžÖŠČÜŅŗµÄpHŌŚ5.5~6.0Ö®¼ä£¬Éś²śŠ§ĀŹ½Ļøß”£µ±æŲÖĘŅ»¶ØĮ÷ĮæµÄĪ²ĘųŹ±£¬µ÷½ŚČÜŅŗµÄpHµÄ·½·ØŹĒ________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ373KŹ±£¬Ä³ 1LĆܱÕČŻĘ÷ÖŠ¼ÓČė2mol NH3·¢ÉśČēĻĀæÉÄę·“Ó¦£ŗ2NH3£Øg£©![]() N2£Øg£©+3H2£Øg£©”£ĘäÖŠĪļÖŹH2µÄĪļÖŹµÄĮæ±ä»ÆČēĻĀĶ¼ĖłŹ¾”£
N2£Øg£©+3H2£Øg£©”£ĘäÖŠĪļÖŹH2µÄĪļÖŹµÄĮæ±ä»ÆČēĻĀĶ¼ĖłŹ¾”£
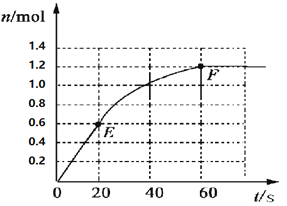
£Ø1£©Ē°20 sÄŚNH3£Øg£©µÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ___________£»
£Ø2£©373KŹ±øĆ·“Ó¦µÄĘ½ŗā³£ŹżµÄÖµĪŖ______________£»
£Ø3£©ČōŌŚ“ĖĘ½ŗāĢåĻµÖŠŌŁ¼ÓČė1molµÄNH3£¬ÓėŌĘ½ŗā±Č½Ļ£¬ŠĀĘ½ŗāŹ±NH3µÄ×Ŗ»ÆĀŹ______£ØĢī”°Ōö“ó”±»ņ”°¼õŠ””±£¬ĻĀĶ¬£©£¬NH3µÄĘ½ŗāÅضČ_________”£
£Ø4£©½«ŌĘ½ŗāÉżĪĀÖĮ473K£¬ÖŲŠĀ“ļĘ½ŗāŹ±£ØĘäĖūĢõ¼ž²»±ä£©£¬H2µÄĘ½ŗāÅضČĪŖNH3µÄ2±¶£¬øĆ·“Ó¦µÄÕż·“Ó¦ĪŖ_________£ØĢī”°·ÅČČ·“Ó¦”±»ņ”°ĪüČČ·“Ó¦”±£©£¬ĪŖŌö“óĘ½ŗāĢåĻµÖŠH2µÄĪļÖŹµÄĮ棬ĻĀĮŠ“ėŹ©ÕżČ·µÄŹĒ£ØĘäĖüĢõ¼žĻąĶ¬£©______”£
a£®ÉżøßĪĀ¶Č b£®Ą©“óČŻĘ÷µÄĢå»ż c£®¼ÓČėŗĻŹŹµÄ“߻ƼĮ d£®ŌŁ³äČėN2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æijæĪĶāŠĖȤŠ”×é¶ŌH2O2µÄ·Ö½āĖŁĀŹ×öĮĖČēĻĀŹµŃéĢ½¾æ”£
(1)ĻĀ±ķŹĒøĆŠ”×éŃŠ¾æÓ°Ļģ¹żŃõ»ÆĒā(H2O2)·Ö½āĖŁĀŹµÄŅņĖŲŹ±²É¼ÆµÄŅ»×鏿¾Ż£ŗÓĆ10mL H2O2ÖĘČ”150mLO2ĖłŠčµÄŹ±¼ä(Ćė)
| 30% H2O2 | 15% H2O2 | 10% H2O2 | 5% H2O2 |
ĪŽ“߻ƼĮ”¢²»¼ÓČČ | ¼øŗõ²»·“Ó¦ | ¼øŗõ²»·“Ó¦ | ¼øŗõ²»·“Ó¦ | ¼øŗõ²»·“Ó¦ |
ĪŽ“߻ƼĮ”¢¼ÓČČ | 360 | 480 | 540 | 720 |
MnO2“߻ƼĮ”¢¼ÓČČ | 10 | 25 | 60 | 120 |
¢ŁøĆŃŠ¾æŠ”×éŌŚÉč¼Ę·½°øŹ±”£æ¼ĀĒĮĖÅØ¶Č”¢___________”¢____________µČŅņĖŲ¶Ō¹żŃõ»ÆĒā·Ö½āĖŁĀŹµÄÓ°Ļģ”£
¢Ś“ÓÉĻŹöÓ°Ļģ¹żŃõ»ÆĒā·Ö½āĖŁĀŹµÄŅņĖŲÖŠČĪŃ”Ņ»øö£¬ĖµĆ÷øĆŅņĖŲ¶Ō·Ö½āĖŁĀŹÓŠŗĪÓ°Ļģ£æ_______________________”£
(2)½«ÖŹĮæĻąĶ¬µ«¾Ū¼ÆדĢ¬²»Ķ¬µÄMnO2·Ö±š¼ÓČėµ½5mL 5%(ĆܶČĪŖ1.0g/cm3)µÄĖ«ŃõĖ®ÖŠ£¬²¢ÓĆ“ų»šŠĒµÄľĢõ²āŹŌ”£²ā¶Ø½į¹ūČēĻĀ£ŗ
ŹµŃé ŠņŗÅ | “߻ƼĮ£ØMnO2£© | ²Ł×÷ Ēéæö | ¹Ū²ģ½į¹ū | ·“Ó¦Ķź³É ĖłŠčµÄŹ±¼ä |
A | ·Ūĩד | »ģŗĻ²»Õńµ“ | ¾ēĮŅ·“Ó¦£¬“ų»šŠĒµÄľĢõø“Č¼ | 5span>·ÖÖÓ |
B | æéד | ·“Ó¦½ĻĀż£¬»šŠĒŗģĮĮµ«Ä¾ĢõĪ“ø“Č¼ | 30·ÖÖÓ |
¢Ł Š“³öH2O2·¢Éś·Ö½āµÄ»Æѧ·“Ó¦·½³ĢŹ½________________”£Ēó³öŹµŃéAÖŠH2O2ŌŚ5·ÖÖÓÄŚµÄĘ½¾ł·“Ó¦ĖŁĀŹ________________”££Ø½į¹ū±£ĮōŠ”ŹżµćŗóĮ½Ī»Źż×Ö£©
¢Ś ŹµŃé½į¹ūĖµĆ÷“߻ƼĮ×÷ÓƵēóŠ”Óė____________________ÓŠ¹Ų”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com