
��14�֣�������ѧ��Ӧ����ʽA+BX+Y+H2O����ش�
(1) ��AΪ������ˮ���Σ�B��ǿ�ʵ���ҳ��ô˷�Ӧ�Ʊ�����X��д���÷�Ӧ�Ļ�ѧ����ʽ �������������X�ķ����� ��
(2) ��AΪ��ɫ���壬Y�ǻ���ɫ���壬д��Y �ĵ���ʽ ���÷�Ӧ�����ӷ���ʽ �� ��������Yͨ��NaOH��Һ�м��ȷ�����Ӧ��������6.72L(��״��)Y����ʱ��ת��0.5mol���ӣ�д���÷�Ӧ�Ļ�ѧ����ʽ ��
(3) ��AΪ�������ʣ�B��ϡ���ᣬ��A��B�����ʵ���֮��Ϊ1:4���з�Ӧ��������ɫ����X��������������Ϊ����ɫ����A������Y��Һ�С�
��д���÷�Ӧ�Ļ�ѧ����ʽ ��
�ں�0.5molY����Һ�ܽ���һ����A������Һ�����ֽ��������ӵ����ʵ���ǡ����ȣ���ԭ��Y�� mol��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
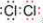
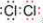
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�������ѧ��Ӧ����ʽA+BX+Y+H2O����ش�
(1) ��AΪ������ˮ���Σ�B��ǿ�ʵ���ҳ��ô˷�Ӧ�Ʊ�����X��д���÷�Ӧ�Ļ�ѧ����ʽ �������������X�ķ����� ��
(2) ��AΪ��ɫ���壬Y�ǻ���ɫ���壬д��Y �ĵ���ʽ ���÷�Ӧ�����ӷ���ʽ �� ��������Yͨ��NaOH��Һ�м��ȷ�����Ӧ��������6.72L(��״��)Y����ʱ��ת��0.5mol���ӣ�д���÷�Ӧ�Ļ�ѧ����ʽ ��
(3) ��AΪ�������ʣ�B��ϡ���ᣬ��A��B�����ʵ���֮��Ϊ1:4���з�Ӧ��������ɫ����X��������������Ϊ����ɫ����A������Y��Һ�С�
��д���÷�Ӧ�Ļ�ѧ����ʽ ��
�ں�0.5molY����Һ�ܽ���һ����A������Һ�����ֽ��������ӵ����ʵ���ǡ����ȣ���ԭ��Y�� mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ��У�������������ۺ��Ծ�����ѧ���֣� ���ͣ������
��14�֣�������ѧ��Ӧ����ʽA+B X+Y+H2O����ش�
X+Y+H2O����ش�
(1) ��AΪ������ˮ���Σ�B��ǿ�ʵ���ҳ��ô˷�Ӧ�Ʊ�����X��д���÷�Ӧ�Ļ�ѧ����ʽ �������������X�ķ����� ��
(2) ��AΪ��ɫ���壬Y�ǻ���ɫ���壬д��Y �ĵ���ʽ ���÷�Ӧ�����ӷ���ʽ�� ��������Yͨ��NaOH��Һ�м��ȷ�����Ӧ��������6.72L(��״��)Y����ʱ��ת��0.5mol���ӣ�д���÷�Ӧ�Ļ�ѧ����ʽ ��
(3) ��AΪ�������ʣ�B��ϡ���ᣬ��A��B�����ʵ���֮��Ϊ1:4���з�Ӧ��������ɫ����X��������������Ϊ����ɫ����A������Y��Һ�С�
��д���÷�Ӧ�Ļ�ѧ����ʽ ��
�ں�0.5molY����Һ�ܽ���һ����A������Һ�����ֽ��������ӵ����ʵ���ǡ����ȣ���ԭ��Y�� mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�걱���в�ƽ���������ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com