
��12�֣�ijУ��̽��С��ͬѧ���о�����ͭ�ܷ�������ص����ȷֽⷴӦ������������ã���������µĶԱ�ʵ��Ͳ������裺
����ȡ��������أ��ֱ������֧�Թܣ�������һ���м��뾭��ȷ������n g����ͭ��ĩ��
�ڽ����������ͬʱ����ͬ�������¼��ȣ����ų�������ͨ��ˮ�У��۲�ų�����Ŀ�����
��ֹͣ���ȡ���ȴ����ԭ�Ȼ�������ͭ�ķ�Ӧ��Ļ�����ˮ�ܽ⣬С�Ĺ��ˣ����˳���������ϴ�Ӳ����
�ܹ۲��˳��������ʵ�״̬����ɫ��
�ݽ��˳��������ʺ�̿�ۻ�ϣ����ܱ������м��ȣ�������Ӧ����������ͨ�����ʯ��ˮ�У����۲�ʵ������
��1���������й�ʵ�鲽��������пɷ��ֲ�������������һЩȱ�ݣ���ָ�������ԸĽ���
______________________________________________________________________��
______________________________________________________________________��
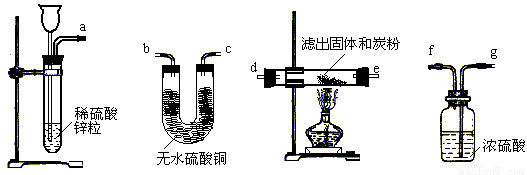
��2�����˻�����һ�ַ��������������ڢݲ����������ͼ��ѡ�����������ӳ�һ��ʵ��װ�ã�����ʵ��װ�õ�����˳����_______��______��________��______��________��________��_______�������ķ���������ң���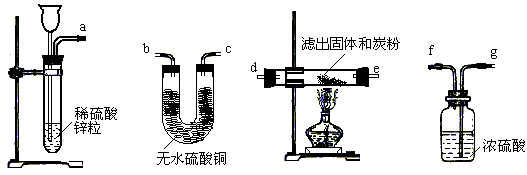

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��I���ζ��������ǻ�ѧ�������е���Ҫ��������֮һ��
��I���ζ��������ǻ�ѧ�������е���Ҫ��������֮һ��| �ζ����� ʵ�����ݣ�mL�� |
1 | 2 | 3 | 4 |
| V����Ʒ�� | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH�������ģ� | 15.95 | 15.00 | 15.05 | 14.95 |

| ʵ�鲽�� | ʵ������ | ʵ����� | |
| ʵ��һ | �������ǵ�ľ������װ�й���������Һ���Թ��� | ľ������ȼ | �����¹���������Һ���ֽ⣨��ֽ���٣� �� �� |
| ʵ��� | ��װ��H2O2��Һ���Թ��м�������Al2O3��Ȼ�����ǵ�ľ�������Թ��� | ľ����ȼ | Al2O3�ܼӿ�H2O2��Һ�ķֽ����� Al2O3�ܼӿ�H2O2��Һ�ķֽ����� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���߿��ܸ�ϰȫ�⡡��ѧ��һ�ָ�ϰ�����γ̡�(�˽�ʵ���) �˽�ʵ��� ���ͣ�043
2003��6�£�������Ч��Ĺ�г���һ��ͭ��(һ�־ƾ�)�����渽������ɫ�������ʣ����Ӿ����˱ǣ���ʢ��26 kg����ɫҺ�壬ר���϶���2000����ǰ�ġ��������ơ��������ҹ����Ž硢��ƽ��һ���ش��֣�
(1)�������Գ����ġ��������ơ�����̽��������̽��һ��������________��
(2)��������������ijУ��ѧ��ȤС��ͬѧ��ע�������ռ�����ͭ���������ɫ�������̽����
������⣺ͭ�����渽����ɫ��������������ЩԪ����ɵģ�
���룺����������Ϻ�����ɫ�������ʿ�����ͭ�̣�
ʵ�鲽�裺

�ٶ��Թ��ڵ���ɫ������м��ȣ�����ȫ�ֽ⣮�۲쵽Aװ������ɫ������ɺ�ɫ��Bװ������ˮ����ͭ�����ɫ��Cװ���г���ʯ��ˮ����ǣ�
��ȡ�������Ⱥ����ɵĺ�ɫ�������Թ��У�����ϡ���ᣮ�۲쵽��ɫ�������ܽ⣬��Һ�����ɫ��
��ȡ����������ɫ��Һ���Թ��У�����һ���ྻ����˿���۲쵽��˿�����к�ɫ����������
ʵ����ۣ���ɫ���������к���________��________��________��________��Ԫ�أ�(��ʾ��װ���ڵĿ������غ��Բ���)�����뽻������ͼ�б���a��b�����������ǣ�a________��b________��
������ʵ�鲽����з�����Ӧ�Ļ�ѧ����ʽΪ________��
�۷�Ӧ��ɺ��������ȥ�ƾ��ƣ����ܳ��ֵ�������________��
�������B��C��װ�öԵ�����Ϊʲô��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���Ĵ�ʡ�߶���������Ի�ѧ�Ծ��������棩 ���ͣ������
(9��)ijУ�о���ѧϰС���ͬѧѧϰ�굪���й����ʵ�����֮�Ե�Ԫ�ص��⻯��NH3���ʵ�̽����
(1)ʵ������ȡ�����Ļ�ѧ����ʽΪ ��
(2)ijͬѧģ���ű���ʳ��ˮ�ռ������ķ����������ű����Ȼ����Һ�ķ����ռ�����������Ϊ���ܷ�ﵽĿ�ģ� (��ܡ���)�������� ��
(3)��С���ͬѧ�������ͼ��ʾ��ʵ��װ��(�гּ�β������װ��δ����)��̽�������Ļ�ԭ�ԡ�
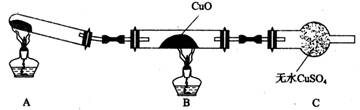
�ٸ�װ�����������һ��ȱ�ݡ�Ϊ��֤ʵ������ȷ�ԣ��Ը�װ�õĸĽ���ʩ��
��
�����øĽ����װ�ý���ʵ�飬CuO��Ϊ��ɫ���ʣ���ˮCuSO4������ͬʱ����һ������Ⱦ�����塣������CuO��Ӧ�Ļ�ѧ����ʽΪ ��
����ͬѧ��ΪNH3��CuO��Ӧ���ɵĺ�ɫ�����п��ܺ�Cu2O��Cu2O��������Һ��Cu+�绯����Cu��Cu2+�������һ����ʵ�����ú�ɫ�������Ƿ���Cu2O
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ӱ�ʡ�߿���ϰ�ۺϲ��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��12�֣�ijУ��̽��С��ͬѧ���о�����ͭ�ܷ�������ص����ȷֽⷴӦ������������ã���������µĶԱ�ʵ��Ͳ������裺
����ȡ��������أ��ֱ������֧�Թܣ�������һ���м��뾭��ȷ������n g����ͭ��ĩ��
�ڽ����������ͬʱ����ͬ�������¼��ȣ����ų�������ͨ��ˮ�У��۲�ų�����Ŀ�����
��ֹͣ���ȡ���ȴ����ԭ�Ȼ�������ͭ�ķ�Ӧ��Ļ�����ˮ�ܽ⣬С�Ĺ��ˣ����˳���������ϴ�Ӳ����
�ܹ۲��˳��������ʵ�״̬����ɫ��
�ݽ��˳��������ʺ�̿�ۻ�ϣ����ܱ������м��ȣ�������Ӧ����������ͨ�����ʯ��ˮ�У����۲�ʵ������
��1���������й�ʵ�鲽��������пɷ��ֲ�������������һЩȱ�ݣ���ָ�������ԸĽ���
______________________________________________________________________��
______________________________________________________________________��
��2�����˻�����һ�ַ��������������ڢݲ����������ͼ��ѡ�����������ӳ�һ��ʵ��װ�ã�����ʵ��װ�õ�����˳����_______��______��________��______��________��________��_______�������ķ���������ң���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com