


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �������ܵ���ʵ�Ksp��25�棩 | |
| �����ܽ�ƽ�� | Ksp |
AgBr��s�� Ag+��aq��+Br-��aq�� | 5��0��10-13mol2?L-2 |
AgI��s�� Ag+��aq��+I-��aq�� | 8.3��10-17mol2?L-2 |
FeS��s�� Fe2+��aq��+S2-��aq�� | 6.3��10-18mol2?L-2 |
ZnS��s�� Zn2+��aq��+S2-��aq�� | 1.6��10-24mol2?L-2 |
CuS��s�� Cu2+��aq��+S2-��aq�� | 1.3��10-36mol2?L-2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Fe��������Br2��Ӧ�����Ƶô�����FeBr3����Fe������I2������ӦҲ�����Ƶô�����FeI3 |
| B��CO2����ͨ��Ca��ClO��2��Һ�п��Եõ�HClO����SO2����ͨ��Ca��ClO��2��Һ��Ҳһ���ܵõ�HClO |
| C��FeCl3��Һ�������ɺ�ò���FeCl3����Fe2��SO4��3��Һ�������ɺ�Ҳ�ò���Fe2��SO4��3 |
| D��Cu��OH��2������HNO3��Һ����Al��OH��3Ҳ������HNO3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
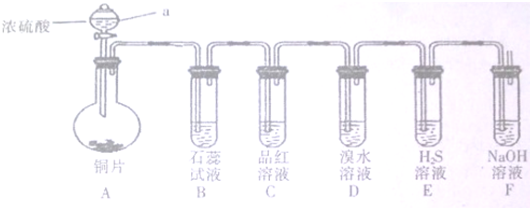
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com