
����Ŀ��NiԪ�������������������Ź㷺��Ӧ�á��ش���������:
(1)��̬Niԭ�Ӽ۲���ӵ��Ų�ʽΪ_______��
(2)��ѧ�����о�������������ֵĹ����У�������Cu-Ni-Fe�ȶ��ֽ��������ȷ��ij�ֽ����������Ǿ��廹�ǷǾ�����ɿ��Ŀ�ѧ�����ǶԹ������______��
(3)Ni������±��(SCN)2��Ӧ����Ni(SCN)2��Ni(SCN)2�У���һ����������Ԫ����____��(SCN)2�����У���ԭ�ӵ��ӻ���ʽ��___��������������Ŀ֮��Ϊ_____��
(4)[Ni(NH3)6](NO3)2�У������ڵĻ�ѧ��Ϊ_____(����)��
a�����Ӽ� b�������� c����λ�� d�����
(5)����CO��N2���۷е�ߵ�ԭ��___��
(6)���Ͻ�����о���ȡ�úܴ��չ��
��ͼ����һ�������Ͻ����ľ����ṹʾ��ͼ���úϽ����1 mol La�ĺϽ������H2����ĿΪ___��
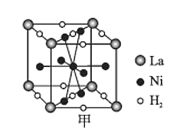
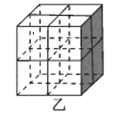
��Mg2NiH4��һ������Ľ����⻯���Mg2NiH4�����У�Niԭ��ռ����ͼ�ҵĶ�������ģ�Mg2+������ͼ�˸�С����������ġ�Mg2+λ��Niԭ���γɵ�___�������������϶�������������϶��������������ܶ�Ϊd g/cm3��Mg2NiH4��Ħ������ΪM g/mol����Mg2+��Niԭ�ӵ���̾���Ϊ___nm(�ú�d��M��NA�Ĵ���ʽ��ʾ)��
���𰸡�3d84s2 X-��������ʵ�� N sp3�ӻ� 5��4 b ����ͬ���ڷ��Ӿ��壬����Է���������ͬ��CO�ļ��Դ���N2�����Է��»��������۷е���� 3NA �������϶ 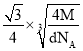 ��107
��107
��������
(1)Ni��28��Ԫ�أ����ݹ���ԭ���ɵ����������Ų�ʽ�������ɵ���۲���ӵ��Ų�ʽ��
(2)����X-��������ʵ��ȷ������Ĵ��ڣ�
(3)Ԫ�صķǽ�����Խǿ����縺�Ծ�Խ��(SCN)2���ӽṹ��ʽΪN��C-S-S-C��N������Sԭ�Ӽ۲���ӶԸ�����4�Һ���2���µ��Ӷԣ����ݼ۲���ӶԻ��������ж���ԭ�ӵ��ӻ���ʽ�����۵���Ϊ����������˫���к���һ��������һ�����������������к���1��������2��������
(4)����λ�������������ӻ�����������Ӽ������������к���λ�������ۼ���������ǻ�ѧ����
(5)���ݼ��Է��ӵķ��Ӽ��������ȷǼ��Է��ӵķ���֮���������������
(6)�ٸ��ݾ�����ԭ�ӷ�̯������La��Ni��H2��Ŀ��д����ѧʽ���ɽ��
��Niԭ��ռ�ݾ����Ķ�������ģ��������ֳ�8��С�����壬Mg2+������ͼ�˸�С����������ģ���λ��Niԭ���γɵ��������϶�ڣ�����Mg2+��Niԭ�ӵ���̾���Ϊ������Խ��ߵ��ķ�֮һ�������ܶȹ�ʽ���㾧���ı߳����ɽ��
(1)28��Ԫ��Ni�ĺ�������Ų�ʽ��1s22s22p63s23p63d84s2���ڲμӷ�Ӧʱ��Niԭ�ӵ�������4s���Ӻʹ�����3d���Ӷ����ܷ����仯�������۲���ӵ��Ų�ʽ��3d84s2��
(2)ȷ��ij�ֽ����������Ǿ��廹�ǷǾ�����ɿ��Ŀ�ѧ�����ǶԹ������X-��������ʵ�飻
(3)��Ni(SCN)2���漰����Ԫ����Ni��S��C��N����Ԫ�أ�����Ni�ǽ���Ԫ�أ��縺����С�������ַǽ���Ԫ��S��C��N�У�Ԫ�طǽ�������ǿ��Ԫ����N������NԪ�صĵ縺�����(SCN)2���ӽṹ��ʽΪN��C-S-S-C��N��Sԭ�Ӽ۲���ӶԸ�����4�Һ���2���µ��Ӷԣ����ݼ۲���ӶԻ������ۿ�֪��ԭ�ӵ��ӻ���ʽ��sp3�ӻ������ڹ��۵���Ϊ����������˫���к���һ��������һ�����������������к���1��������2����������(SCN)2�����к��е�������Ŀ��5����������Ŀ��4��������������������Ŀ֮��Ϊ5��4��
(4)[Ni(NH3)6](NO3)2�����ӻ����������[Ni(NH3)6]2+��NO3-ͨ�����Ӽ���ϣ���������[Ni(NH3)6]2+�У�����Ni2+��������λ��NH3֮��ͨ����λ����ϣ�����λ��NH3�У�N��H����Ԫ�ص�ԭ��֮��ͨ�����ۼ�N-H��ϣ������в�����������������ǻ�ѧ�������Բ����ڵĻ�ѧ��ѡ��Ϊb��
(5) CO��N2�����ڷ��Ӿ��壬����Է���������ͬ������CO�Ǽ��Է��ӣ�N2�ǷǼ��Է��ӣ�CO�ļ��Դ���N2�����Է��»����������CO�۷е��N2���ߣ�
(6)�پ����У�La����Ϊ8��![]() =1��Ni����Ϊ8��
=1��Ni����Ϊ8��![]() +1=5��H2����Ϊ2��
+1=5��H2����Ϊ2��![]() +8��
+8��![]() =3�������ʻ�ѧʽΪLaNi5(H2)3������1 mol La�ĺϽ������3 mol H2�����е�H2����ĿΪ3NA��
=3�������ʻ�ѧʽΪLaNi5(H2)3������1 mol La�ĺϽ������3 mol H2�����е�H2����ĿΪ3NA��
��Niԭ��ռ�ݾ����Ķ�������ģ��������ֳ�8��С�����壬Mg2+������ͼ�˸�С����������ģ���λ��Niԭ���γɵ��������϶�ڣ�����Mg2+��Niԭ�ӵ���̾���xΪ������Խ��ߵ��ķ�֮һ���辧���߳�Ϊa cm����x=![]() a cm����������8��Mg2+������������4����Mg2NiH4������������m=
a cm����������8��Mg2+������������4����Mg2NiH4������������m=![]() =da3g��a=
=da3g��a= cm����x=
cm����x=![]() a cm=
a cm=![]() ��
��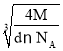 cm=
cm=![]() ��
��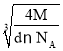 ��107nm��
��107nm��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ᵪ���ǵ������ҩ�Ĵ������ϳ�·����ͼ��ʾ��
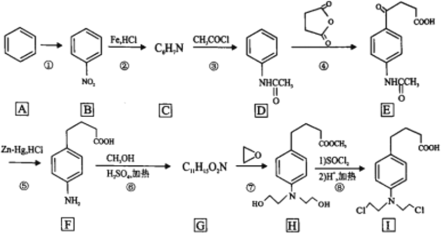
�ش��������⣺
(1)��Ӧ��������Լ���������______________��B�еĹ�����������______________
(2)C�Ľṹ��ʽΪ______________��
(3)д�����б����ṹ�����ܷ���������Ӧ���ܷ���ˮ�ⷴӦ��D��ͬ���칹��Ľṹ��ʽ______________��(�����������칹��ֻ��д��3��)
(4)�ڵķ�Ӧ������______________��
(5)д��F��G�ķ�Ӧ����ʽ______________��
(6)����ɱ��� �Ʊ�
�Ʊ� �ĺϳ�·��(���Լ���ѡ)��______________
�ĺϳ�·��(���Լ���ѡ)��______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
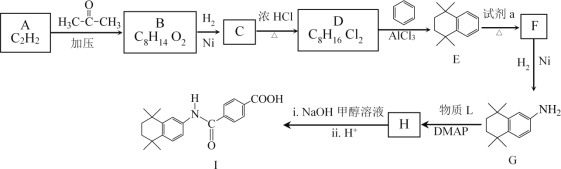
����Ŀ�����װ���͡(I)���������Ƽ���Ѫ������ϳ��о�������Ҫ���壬�ϳ�·����ͼ��ʾ��
��֪��i.HC![]() CH+
CH+
ii.R��NO2![]() R��NH2
R��NH2
iii.R1��NH2+![]() +HCl
+HCl
(1)A�����������_________��
(2)B�Ľṹ��ʽ��________��
(3)D��E�Ļ�ѧ����ʽ��__________��
(4)�Լ�a��_________��
(5)��֪H�ںϳ�I��ͬʱ�������ɼ״���G��H��������L�Ľṹ��ʽ��______��
(6)B��һ��ͬ���칹�����������������ṹ��ʽ��________��
���ܷ���������Ӧ
�ں˴Ź�������ֻ���������շ�
(7)D��E�Ĺ������ж��ָ�����������ڸ߷��ӻ�����Ľṹ��ʽ��_______��
(8)![]() Ҳ�Ǻϳ����װ���͡(I)��һ��ԭ�ϣ��ϳ�·����ͼ��ʾ����������������Ϣ���м����Ľṹ��ʽ��_______________��
Ҳ�Ǻϳ����װ���͡(I)��һ��ԭ�ϣ��ϳ�·����ͼ��ʾ����������������Ϣ���м����Ľṹ��ʽ��_______________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ������˵������ȷ����
A. 6.02��1023���ǰ����ӵ�����
B. 1 molˮ�е���ԭ����ĿΪ2NA
C. �����ӵ����������ӵ����ʵ�����1 mol
D. 1 mol ��������ԭ����ԼΪ2.408��1024��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʾ��Һ��Ũ�ȵķ���ͨ�������֣���Һ�����ʵ���������(w)�����ʵ���Ũ��(c)�������������Һʱ�����ݲ�ͬ����Ҫ���в�ͬ�����Ʒ������������ա�
(1)��10%(�ܶ�Ϊ1.01 g��cm��3)������������Һ���Ƴ�27.5 g 2% ������������Һ��
�ټ��㣺��________g 10%(�ܶ�Ϊ1.01 g��cm3)������������Һ�������Ϊ________mL�����________mLˮ(��ˮ��1 g��cm��3)����ϡ�͡�
����ȡ����________mL��Ͳȡ10% �������ƣ���ȡʱ����Ҫ����Ͳ________����ˮƽ��Ȼ�����ձ����________mL��Ͳ��ȡ����ˮҲע���ձ��
���ܽ⣺��________��������Һ������ȣ�����27.5 g 2% ������������Һ��
(2)��98%(�ܶ�Ϊ1.84 g��cm��3)��Ũ����ϡ�ͳ�3 mol��L��1��ϡ����100 mL���ش��������⣺
����ҪȡŨ����________mL��
�����Ʋ����ɷֽ�����¼�����������ȷ�IJ���˳����__________________________(����ĸ����ͬ)��
A��������ƿ��ע����������ˮ������Ƿ�©ˮ
B������������ˮϴ���ձ���������������Һע������ƿ�����ظ���������
C��������ȴ��ϡ����ע���Ѽ�鲻©ˮ������ƿ��
D�����ݼ��㣬����Ͳ��ȡһ�������Ũ����
E����Ũ�������ձ�������ע��ʢ������ˮ��С�ձ��У��������ò���������
F����������ƿ���ӣ���ҡ��
G���ý�ͷ�ιܵμ�����ˮ��ʹ��Һ����ǡ����̶�������
H������������ƿ��С�ĵؼ�����ˮ��ʹҺ��ӽ��̶���
(3)ʵ����������1 mol��L��1������������Һ��1 mol��L��1��������Һ��100mL��
��Ҫ��������������Һ������������ƽ��ȡ�������ƹ���ʱ����ƽ����Ϊ________��
A��4.0 g����������B��4.00 g�������� C����4.0 g
������������������Һ��������Һ�ĸ��������У������Բ�ͬ����__________��
A����������ȡ������B���ܽ��ϡ�� ��C����Һ��ϴ�ӡ���D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���衢��(32Ge���۵� 937��)����(31Ga)������Ҫ�İ뵼����ϣ��ں��պ����ء�������̽�⡢����ͨѶ�������ѧ��̫���ܵ�ء���ѧ����������ҽѧ�������й㷺����Ҫ��Ӧ�á��������ͬ����Ԫ�ء�
(1)����Ԫ�����ڱ��е�λ����________��
(2)���������Ԫ�ض����γ��Ȼ��� RCl4(R����Si��Ge)����ԭ�ӽṹ�ǶȽ���ԭ�� _______��
(3)��Ȼ���ʯ����Ũ�ȷdz��ͣ���˴���ӹ�����(������̬��)�л�������һ�ַdz���Ҫ�ķ�������ͼ��һ����ȡ������̣�
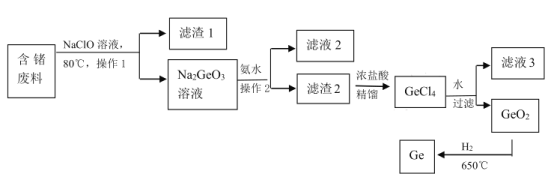
��NaClO ��Һ��ȡ��������е���ʱ������Ӧ�����ӷ���ʽΪ_____��Ϊ�˼�NaClO ��Һ��ȡ������ϵ����ʣ����Բ�ȡ�Ĵ�ʩ��_____��
�ڲ��� 1 �Ͳ��� 2 ��____��
�� GeO2 ���۵�Ϊ 1086�棬����������ԭGeO2��ÿ���� 146kg ����ų� akJ ���������÷�Ӧ���Ȼ�ѧ����ʽΪ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�����ӷ���ʽ��ȷ����
A.��Ca(ClO)2��Һ��ͨ�����CO2�ƴ����2ClO + H2O + CO2=2HClO +![]()
B.[Ag(NH3)2]OH���Ũ���ᷴӦ����AgCl��[Ag(NH3)2]+ + OH + 3H+ + Cl=AgCl��+2![]() + H2O
+ H2O
C.Cl2���ȵ�NaOH��Һ��Ӧ��ȡNaClO3��2Cl2 + 6OH![]() 3Cl +
3Cl +![]() + 3H2O
+ 3H2O
D.������KMnO4��Һ��ͨ��SO2��2![]() + 5SO2 + 4OH=2Mn2+ + 5
+ 5SO2 + 4OH=2Mn2+ + 5![]() + 2H2O
+ 2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������(FeC2O4��2H2O��M=180g/mol)�ʵ���ɫ,������ɹ����ͼ��ijʵ��С����������һϵ��̽����
I.�����������������ȷֽ�����̽��
(1)�������ɷֵ�̽����С���Ա��������װ�ÿ��ظ�ѡ��)����ʵ�飺

��E��ʢװ��ʯ�ҵ���������Ϊ_________��
�ڰ������������ҵķ�������װ�õĽӿ�˳��Ϊa��g��f��_____��β������װ��(�������ظ�ʹ��)��
��ʵ��ǰ��ͨ��һ��ʱ��N2����Ŀ��Ϊ__________________��
��ʵ��֤������������к���CO�����ݵ�ʵ������Ϊ_____________��
(2)С���Ա���ʵ��֤����A�зֽ��Ĺ���ɷ�ΪFeO���������������ֽ�Ļ�ѧ����ʽΪ____________________��
(3)ɹ����ͼʱ����K3[Fe(CN)6]��ҺΪ��ɫ�����÷�Ӧ�Ļ�ѧ����ʽΪ______________��
��.��������������Ʒ���ȵIJⶨ
��ҵ�ƵõIJ������������г�����FeSO4���ʣ��ⶨ�䴿�ȵIJ������£�
����1����ȡmg��������������Ʒ������ϡHSO4�У����250mL��Һ��
����2��ȡ������Һ25.00mL���� cmol/L KMnO4��Һ�ζ����յ㣬���ı�ҺV1mL��
����3����Ӧ����Һ�м�������п�ۣ���ַ�Ӧ��������ϡH2SO4������ cmol/L KMnO4����Һ�ζ����յ㣬���ı�ҺV2mL��
(4)����2�еζ��յ������Ϊ______________������3�м���п�۵�Ŀ��Ϊ_______��
(5)��������������Ʒ�Ĵ���Ϊ________��������1������Һʱ����Fe2+���������ʣ���ⶨ�����____(����ƫ��������ƫ��������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ƿ�չ�߿Ƽ���ҵ���������˼������ɻ�ȱ��ԭ�ϡ�H2TeO3��һ�ֱȲ����������Ķ�Ԫ�ᣬ��ҵ�ϳ���ͭ������[��Ҫ�ɷ����ڻ���ͭ(Cu2Te)����������Ag��Au]�����ڣ��乤���������£�
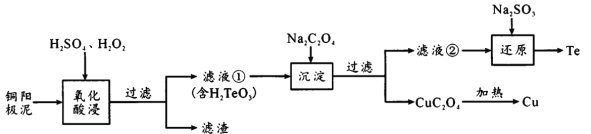
��֪��CuC2O4��KspΪ2.2��10-8������Ũ��С��1��10-5mol/Lʱ����������ȫ������
(1)Cu2Te��Te�Ļ��ϼ���___��
(2)�����ijɷ���___����Һ���к��е��������ʱ��������Ϊ____���������ʱ�¶ȹ���ʹ�ڵĽ����ʽ��ͣ�ԭ����_______��
(3)��ҪʹCu2+��ȫ������Ӧ����C2O42-��Ũ�Ȳ�����_____��
(4)��ԭ��Ӧ�����ӷ���ʽΪ______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com