
(1)��4�֣�����ͼ��ʾA��B��C��D����������
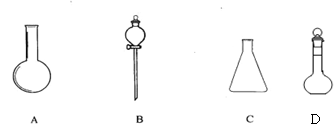 |
��ѡ�������������Ƶ���ţ�������Ӧ�Ŀո��ڣ�
���ձ� ����ͨ©�� ��Բ����ƿ ����ƿ �ݷ�Һ©�� ������ƿ
A ______________ B______________C______________D______________
(2)(4��)ʵ���Ҽ���ij��Һ���Ƿ���SO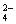 �IJ��������ǣ�
�IJ��������ǣ�
��
ʵ���Ҽ���ij��Һ���Ƿ���Cl���IJ��������ǣ�
��
(3)(4��)ѡ������ʵ�鷽���������ʣ������뷽����������ں����ϡ�
A����ȡ��Һ B������ C���ᾧ D����Һ E������ F������
��1����������л��е���ɳ______�� ��2��������ˮ�Ļ����____ __��
��3������ˮ�����͵Ļ����______�� ��4������ƾ���ˮ�Ļ����______��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ��U�ιܵ���˱�ˮ�ͽ�������м��������(�����Ϊ1��4)�Ļ�����壬�ٶ�������ˮ���ܽ�ȿ��Ժ��ԡ�������м���������Ļ�������װ�÷������й����ĵط����û�����建���ط�Ӧһ��ʱ�䡣
(1)���������������Ӧ��֣���ֻ����һ���л����д����ѧ����ʽ��________________________________________________________________________��
(2)��������Сʱ�ķ�Ӧ��U�ι��Ҷ˵IJ�������ˮ���仯��______��
A�����ߡ��������������� B������
C������ D����ȷ��
(3)�Խ���U�ι��Ҷ˵IJ�������ˮ���仯��ԭ��
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1 mol��L��1 H2SO4�����(����)
A��1 Lˮ�к���1 mol H2SO4
B��1 L��Һ�к���1 mol H��
C����98 g H2SO4����1 Lˮ�������Һ
D��ָ1 L H2SO4��Һ�к�98 g H2SO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ˮ�Dz�������������,Ϊ�˼���Cl-�Ĵ���,���ѡ�����������еģ� ��
A��ʯ����Һ B�����Ȼ�̼ C������������Һ D����������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
0.5L 1mol/L��FeCl3��Һ��0.2L 1 mol/L��KCl��Һ�У�Cl�������ʵ���Ũ��֮��Ϊ�� ��
A��15��2 B��1��1 C��3��1 D��1��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й������ʵ�Ԫ����ɻ�����˵����ȷ����
A�������Ԫ�� B���ᶼ����Ԫ��
C���ζ�������Ԫ�� D��Ư�۾���Һ�ȡ��ɱ���Ϊ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʼ��ÿ��ת������ͨ��һ����Ӧʵ�ֵ���
��Fe��Fe2O3��Fe(OH)3 �� Na��Na2O2��Na2CO3
�� S��SO3��H2SO4��MgSO4 �� C��CO��CO2��Na2CO3
A���٢� B���ۢ� C���ڢ� D���٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�Ͽ�������������Ʊ����Ʊ���������2Al2(SO4)3��3S  2Al2O3��9SO2���������й�˵������ȷ����(����)
2Al2O3��9SO2���������й�˵������ȷ����(����)
A����Ӧ��Al2(SO4)3������
B��Al2O3����������
C���÷�Ӧ�У�ÿת��0.3 mol��������5.04 L SO2
D�����������뻹ԭ���������֮��Ϊ1�U2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
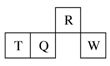
������Ԫ��R��T��Q��W��Ԫ�����ڱ��е����λ������ͼ��ʾ������T������������������������ȡ������жϲ���ȷ����
A�������̬�⻯������ȶ��ԣ�R>Q
B��ԭ�Ӱ뾶��T>Q>R
C������������Ӧˮ��������ԣ�Q<W
D����T������Һһ��������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com