
��ij��Ӧ��ϵ�д����������ʣ�Cr3+��S2O82����Cr2O72����H+��H2O��SO42������֪�ڸ÷�Ӧ��������Ӧֻ��Cr3+��Cr2O72������Ӧ��������ҪAg+�������������ȡ���ش��������⣺
��1����֪S2O82�������д���һ������������O��O��������S2O82����������Ԫ�صĻ��ϼ�Ϊ__________�ۡ�
��2��д���÷�Ӧ��ϵ�з�����Ӧ�����ӷ���ʽ��_______________________________��
��3�����������μӷ�Ӧ���ı䷴Ӧ;�������������ӷ���ʽ��ʾAg+�Ĵ����̣�____________________________��________________________��
��4������������Ӧ��Ƴ�һ��ԭ��أ���ʯī���缫�����为����ӦʽΪ��_____________________��������ӦʽΪ��____________________________��
��1��+6��2�֣� ��2��2Cr3++3S2O82��+7H2O![]() Cr2O72��+6SO42��+14H+��3�֣�
Cr2O72��+6SO42��+14H+��3�֣�
��3��2Cr3++6Ag++7H2O=Cr2O72��+6Ag��+14H+��2�֣� 2Ag+S2O82��=2Ag++2SO42����2�֣�
��4��2Cr3++7H2O��6e��= Cr2O72��+14H+��3�֣� 3S2O82��+6e��=6SO42����3�֣�
��������1���������е�������ԭ�Ӿ�Ϊ��1�ۣ�������ԭ�Ӿ�Ϊ��2�ۡ��ݴ˿ɼ����S2O82����������Ԫ�صĻ��ϼ�Ϊ+6�ۡ���2�����ݻ��ϼ�������Ⱥ͵���غ㼴����ɡ�
��3�����ݴ����ĸ��Ag+�Ĵ������ǣ�Ag+����Cr3+��Ӧ����Ag��Cr2O72�������ɵ�Ag����S2O82����Ӧ����Ag+��SO42����
��4����������������Ӧ����Cr3+�ŵ磻����������ԭ��Ӧ����S2O82���ŵ硣


 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| �� |
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ij��Ӧ��ϵ�д����������ʣ�Cr3+��S2O82����Cr2O72����H+��H2O��SO42������֪�ڸ÷�Ӧ��������Ӧֻ��Cr3+��Cr2O72������Ӧ��������ҪAg+�������������ȡ���ش��������⣺
��1����֪S2O82�������д���һ������������O��O��������S2O82����������Ԫ�صĻ��ϼ�Ϊ__________�ۡ�
��2��д���÷�Ӧ��ϵ�з�����Ӧ�����ӷ���ʽ��_______________________________��
��3�����������μӷ�Ӧ���ı䷴Ӧ;�������������ӷ���ʽ��ʾAg+�Ĵ����̣�____________________________��________________________��
��4������������Ӧ��Ƴ�һ��ԭ��أ���ʯī���缫�����为����ӦʽΪ��_____________________��������ӦʽΪ��____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
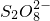 ��
��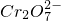 ��H+��H2O��
��H+��H2O��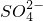 ����֪���÷�Ӧ��������Ӧֻ��Cr3+��Cr2O
����֪���÷�Ӧ��������Ӧֻ��Cr3+��Cr2O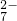 �÷�Ӧ��������ҪAg+�������������ȣ�
�÷�Ӧ��������ҪAg+�������������ȣ�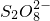 �д���һ����������-O-O-������
�д���һ����������-O-O-������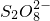 ����Ԫ�صĻ��ϼ�Ϊ______��
����Ԫ�صĻ��ϼ�Ϊ______���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com