
�������������������ʴ��ÿ����ʴ����ʧ�ĸֲ�ռ���������������ķ�֮һ��
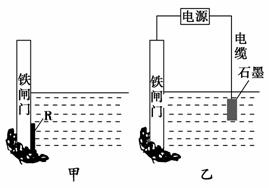
(1)������ʴ��Ҫ��������ʴ���ø�ʴ�����еĵ缫��ӦʽΪ_________
_______________________________________________________________��
(2)Ϊ�˽���ijˮ�����բ�ű���ʴ�����ʣ����Բ���ͼ����ʾ�ķ���������
��������բ���ϵĹ������R���Բ��� (����)��
A��ͭ B���� C��п D��ʯī
(3)ͼ����ʾ�ķ���Ҳ���Խ�����բ�ŵĸ�ʴ���ʣ�������բ��Ӧ��������ֱ
����Դ��________����
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л��������в�ͬ�ķ����������˵����ȷ����(����)
�ٴ����Ԫ�ط֣���������������ڴӷ�����̼�Ǽ���״�֣���״�л��������״�л�������۴ӹ����ŷ֣�ϩ����Ȳ������������±�����������ӡ�ȩ��ͪ�����ᡢ����
A���٢� B���٢� C���٢ڢ� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1 molij��A��1 mol����ȫȼ�գ���A�ȱ�������1 mol O2����A���ӽṹ����֧�����������
(1)��AΪ��״���������������ʵ�����Br2�����ӳɷ�Ӧ����A�Ľṹ��ʽΪ________________________________________________________________________��
(2)��AΪ��״ϩ����1 mol A������2 mol Br2�����ӳɷ�Ӧ��A������ʵ�����Br2�ӳɺ�Ŀ��ܲ���ֻ��2�֣���A�Ľṹ��ʽΪ
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������绯ѧ��ʴ�ص��� (����)��
A����ͭ(ͭп�Ͻ�)������ͭ��ײ���ͭ��
B������������о(�����Ǵ���)��������
C��������������ͭ��������ڽӴ�����������
D�����ʽ��ƾ��ú����䰵
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
д����������йػ�ѧ����ʽ����������ԭ��Ӧ�ı������ת�Ƶķ������Ŀ������������ԭ��Ӧ��˵����Ӧ���͡�
(1)����������������______________________________________________��
(2)����������������������________________________________________��
(3)������������������____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪HF��CH3COOH��Ϊ���ᣬ����ǿ��˳��ΪHF>CH3COOH������˵����ȷ����(����)
A��Ũ�Ⱦ�Ϊ0.1 mol��L��1��NaF��CH3COONa��Һ��Ƚϣ�CH3COONa��Һ���Խ�ǿ
B��0.1 mol��L��1 CH3COOH��Һ����ˮϡ�����У���������Ũ�Ⱦ���С
C��NaF��Һ��ֻ����Na����F����H����OH����H2O������
D��NaF��Һ�м�������NaOH���壬��Һ��c(F��)��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����(����)
A����ϡ��ˮ��μ���ϡ�����У�����ҺpH��7ʱ��c(SO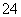 )��c(NH
)��c(NH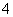 )
)
B�����ִ�����Һ�����ʵ���Ũ�ȷֱ�Ϊc1��c2��pH�ֱ�Ϊa��a��1����c1��10c2
C��pH��11��NaOH��Һ��pH��3�Ĵ�����Һ�������ϣ�����ʯ����Һ�ʺ�ɫ
D����0.1 mol��L��1�İ�ˮ�м�����������粒��壬����Һ��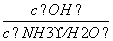 ����
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ᡢ�����̼�������������г��������ʡ����б�����ȷ����(����)
A����NaHCO3��Һ�м�����������ʵ�����NaOH����Һ�е�������ֻ��CO ��OH��
��OH��
B��NaHCO3��Һ�У�c(H��)��c(H2CO3)��c(OH��)
C��10 mL 0.10 mol��L��1 CH3COOH��Һ��������ʵ�����NaOH����Һ�����ӵ�Ũ���ɴ�С��˳���ǣ�c(Na��)>c(CH3COO��)>c(OH��)>c(H��)
D���к������pH����ͬ��HCl��Һ��CH3COOH��Һ�����ĵ�NaOH���ʵ�����ͬ
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com