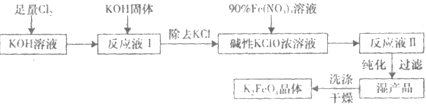
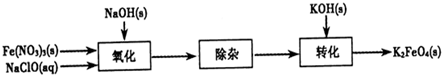
�⣺��1���������������壬�ܺ��ռ���Һ��Ӧ����Ӧʵ��Ϊ��Cl
2+2OH
-=Cl
-+ClO
-+H
2O���ʴ�Ϊ��Cl
2+2OH
-=Cl
-+ClO
-+H
2O��
��2����������ȷ������������Ϊ�Ȼ��ơ����������Լ��������ƵĻ���������Ӧ��֮�����û����II�������NaNO
3��NaCl��NaOH��K
2FeO
4��������K
2FeO
4�õ��ĸ���Ʒ��NaNO
3��NaCl��NaOH������NaNO
3��ըҩ��NaCl������ζƷ���ȼҵԭ�ϵȣ��ʴ�Ϊ��NaNO
3��NaCl��NaOH��NaNO
3��ըҩ��NaCl������ζƷ���ȼҵԭ�ϵȣ�
��3��Ѱ������¶�Ҫ�߱������������¶��·�Ӧ���ʿ죬���ɸ�����صIJ��ʽϴ������棬���Թ�ҵ����������¶�Ϊ26�棬��Ϊ�ڸ��¶������ɸ�����صIJ������ʱFe��NO
3��
3��NaClO������Һ�������Ũ��֮��Ϊ
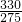
=1.2���ʴ�Ϊ��26��1.2��
��4��ϴ�Ӽ���ѡ��Ҫ��Ҫ��K
2FeO
4ˮ�������������õ��Լ�����ѡ���У�A����K
2FeO
4ˮ�⣬B�д�����ˮ���Լ��ԣ���K
2FeO
4ˮ�������������ã�C��D�е�笠���������������ˮ�⣬��ˮ��������ԣ���K
2FeO
4ˮ�����ٽ����ã���ѡB���ʴ�Ϊ��B��
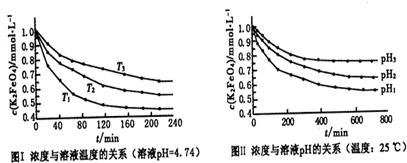
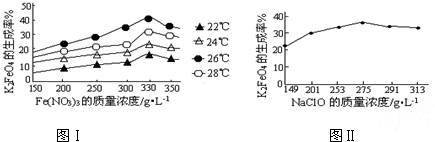
��5���������ֱ����ڲ�ͬ�¶ȵĺ���ˮԡ�У����ⶨc��FeO
42-���ı仯����Ȼ��ȷ���¶ȶ�FeO
42-Ũ�ȵ�Ӱ��������ʴ�Ϊ��̽���¶ȶ�FeO
42-Ũ�ȵ�Ӱ�죻
��6��A����ͬPHֵʱ����Һ����Ԫ�صĴ�����̬����������ͬ��������PHֵ����6ʱ����ֻ��������̬����A����
B����pH=10��������Һ�м�������pH=2��HFeO
4-�ķֲ���������������ּ�С����B����
C��pH=6��������Һ�У���Ԫ�صĴ�����̬��HFeO
4-��FeO
42-����KOH��Һ��ֻ��HFeO
4-�ܷ�Ӧ��������Ӧ�����ӷ���ʽΪ��HFeO
4-+OH
-=FeO
42-+H
2O����C��ȷ��
�ʴ�Ϊ��C��
��������1���������������壬�ܺ��ռ���Һ��Ӧ�����Ծݴ���������84������Һ��
��2����������ȷ�������������Լ�������Ӧ��֮�����û����II����ɣ����з����K
2FeO
4�ͻᷢ�ֵõ��ĸ���Ʒ��ʲô���������ʵ����ʾ�����;��������ʵij�����;���ش�
��3��Ѱ������¶�Ҫ�߱������������¶��·�Ӧ���ʿ죬���ɸ�����صIJ��ʽϴ������棻
��4��ϴ�Ӽ���ѡ��Ҫ��Ҫ��K
2FeO
4ˮ�������������õ��Լ���
��5���������ֱ����ڲ�ͬ�¶ȵĺ���ˮԡ�У����ⶨc��FeO
42-���ı仯����Ȼ��ȷ���¶ȶ�FeO
42-Ũ�ȵ�Ӱ�������
��6������ͼ�������������ͼ�������������壮
������������һ���ۺ�֪ʶ��Ŀ������ѧ�������ͽ��������������ѶȽϴ�

 4Fe��OH��3+8OH-+3O2�������ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��______��Һ������ţ���
4Fe��OH��3+8OH-+3O2�������ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��______��Һ������ţ���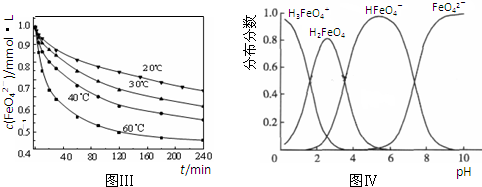
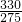 =1.2���ʴ�Ϊ��26��1.2��
=1.2���ʴ�Ϊ��26��1.2��

 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д� �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�