
����Ŀ����2016����ˮ��һ����ĩ���ơ��صĵ⻯���������Ϳ�ѧʵ������ʮ����Ҫ��Ӧ�á���ҵ���õ⡢NaOH����мΪԭ�Ͽ������⻯�ƣ�������������ͼ��ʾ��
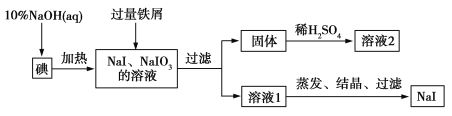
��1��NaOH��Һ�͵ⷴӦʱ��Ҫ�ϸ�����¶ȣ�����¶ȹ��ͣ������ɵ�ĵͼ۸���ƷNaIO����NaOH��Һ�͵ⷴӦʱ������Һ��IO![]() ��IO�������ʵ���֮��Ϊ1��1����÷�Ӧ�����ӷ���ʽΪ______ ��
��IO�������ʵ���֮��Ϊ1��1����÷�Ӧ�����ӷ���ʽΪ______ ��
��2�����������м��������м��Ŀ����__________________���������ù����г�ʣ����м�⣬���к��ɫ���壬�������мʱ������Ӧ�Ļ�ѧ����ʽ��________ __________��
��3����Һ2�г�����H���⣬һ�����е���������______ ____________�������ʵ��֤ʵ�ý��������ӵĴ��ڣ�__________________________________��
��4����Һ2��һϵ��ת�����Եõ�������������(FeC2O4��2H2O)����ȡ3.60 g������������(��Է���������180)�����ط���������ȷֽ⣬�õ�ʣ�������������¶ȱ仯��������ͼ��ʾ��
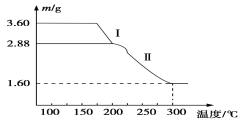
������ͼ�����ݣ�������Ϣд������I�����Ļ�ѧ����ʽ��____________ ______��
��300��ʱʣ�����ֻ��һ�����������������ͨ������ȷ����������Ļ�ѧʽ��________ ________��
���𰸡���1��4I2��8OH��===IO![]() ��6I����4H2O��IO��
��6I����4H2O��IO��
��2����ԭIO![]() (��ʹIO
(��ʹIO![]() ת��ΪI��)(��������)
ת��ΪI��)(��������)
NaIO3��2Fe��3H2O===NaI��2Fe(OH)3��
��3��Fe2�� ȡ����������Һ���Թ�����������������KMnO4��Һ����KMnO4��Һ��ɫ��֤������Fe2��(������K3[Fe(CN)6]������ɫ����)
��4����FeC2O4��2H2O![]() FeC2O4��2H2O��
FeC2O4��2H2O��
��Fe2O3
����������1��NaOH��Һ�͵ⷴӦʱ������Һ��IO![]() ��IO�������ʵ���֮��Ϊ1��1������Ϊ1mol�����ɵ����غ��֪�����ɵ����ӵ����ʵ���Ϊ6mol����ϵ���غ㼰ԭ���غ��֪���ӷ�ӦΪ��4I2��8OH��===IO
��IO�������ʵ���֮��Ϊ1��1������Ϊ1mol�����ɵ����غ��֪�����ɵ����ӵ����ʵ���Ϊ6mol����ϵ���غ㼰ԭ���غ��֪���ӷ�ӦΪ��4I2��8OH��===IO![]() ��6I����4H2O��IO������2��������м��Ŀ���ǻ�ԭIO
��6I����4H2O��IO������2��������м��Ŀ���ǻ�ԭIO![]() ��ʹIO
��ʹIO![]() ת��ΪI����������м�ķ���ʽΪNaIO3��2Fe��3H2O===NaI��2Fe(OH)3������3�������г�ʣ�����м�⣬���к��ɫ���壬��������õ�����Һ2�г�����H���⣬������ȫ�ܽ⣬һ������Fe2����֤�������������ӵķ���Ϊȡ����������Һ���Թ��У�������������KMnO4��Һ����KMnO4��Һ��ɫ��֤������Fe2��(������K3[Fe(CN)6]������ɫ����)����4����3.60 g�����������������ʵ���Ϊ0.2mol������Iʹ����������3.60-2.88=0.72�ˣ�ǡ��Ϊ0.4molˮ�������������I�����ķ�Ӧ����ʽ��FeC2O4��2H2O
ת��ΪI����������м�ķ���ʽΪNaIO3��2Fe��3H2O===NaI��2Fe(OH)3������3�������г�ʣ�����м�⣬���к��ɫ���壬��������õ�����Һ2�г�����H���⣬������ȫ�ܽ⣬һ������Fe2����֤�������������ӵķ���Ϊȡ����������Һ���Թ��У�������������KMnO4��Һ����KMnO4��Һ��ɫ��֤������Fe2��(������K3[Fe(CN)6]������ɫ����)����4����3.60 g�����������������ʵ���Ϊ0.2mol������Iʹ����������3.60-2.88=0.72�ˣ�ǡ��Ϊ0.4molˮ�������������I�����ķ�Ӧ����ʽ��FeC2O4��2H2O![]() FeC2O4��2H2O�������������������е���Ԫ�ص�����Ϊ3.6��56/180=1.12g������������������Ԫ����ȫת�����������У�����������Ԫ�ص�����Ϊ1.60-1.12=0.48g����Ԫ�غ���Ԫ�ص����ʵ���Ϊ1.12/56:0.48/16=2:3,��ΪFe2O3
FeC2O4��2H2O�������������������е���Ԫ�ص�����Ϊ3.6��56/180=1.12g������������������Ԫ����ȫת�����������У�����������Ԫ�ص�����Ϊ1.60-1.12=0.48g����Ԫ�غ���Ԫ�ص����ʵ���Ϊ1.12/56:0.48/16=2:3,��ΪFe2O3



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��Q��R�����ֶ�����Ԫ�أ�ԭ��������������XԪ����YԪ����������������֮�;�Ϊ0��Ԫ��Q��������![]() �����ĵ�����������������ͬ��Z��R�ֱ��ǵؿ��к�����ߵķǽ���Ԫ�غͽ���Ԫ�ء�
�����ĵ�����������������ͬ��Z��R�ֱ��ǵؿ��к�����ߵķǽ���Ԫ�غͽ���Ԫ�ء�
��ش��������⣺
��1������Ԫ��ԭ�ӵİ뾶�ɴ�С��˳���ǣ�дԪ�ط��ţ�____________��
��2��X��Z�γɵ�һ�ֻ�����Ⱥ����Լ��ֺ��Ǽ��Լ�������Ļ�ѧʽ��_________���û�������к�ǿ�������ԣ�д������SO2��Ӧ�Ļ�ѧ����ʽ��____________________���˻�������ijЩ��Ӧ��Ҳ�ɱ��ֻ�ԭ�ԣ�д���������Ը�����ط�Ӧ�����ӷ���ʽ��_______________��
��3��������ijЩԪ����ɵĻ�����A��B��C��D������ת����ϵ��A![]() B����ˮ��Һ�н��У�����C������ˮ�����Ե����壻D�ǻ�ɫ���塣д��D�ĵ���ʽ��_____________�����A��B��������Ԫ����ɣ�BΪ���Բ�������A�Ļ�ѧʽΪ______________����A������C��Ӧ����ΪB�����ӷ���ʽΪ_________________��
B����ˮ��Һ�н��У�����C������ˮ�����Ե����壻D�ǻ�ɫ���塣д��D�ĵ���ʽ��_____________�����A��B��������Ԫ����ɣ�BΪ���Բ�������A�Ļ�ѧʽΪ______________����A������C��Ӧ����ΪB�����ӷ���ʽΪ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2KMnO4+16HCl�T2KCl+2MnCl2+5Cl2��+8H2O��Ӧ���������뻹ԭ�����ʵ���֮��Ϊ�� ��
A.1��8
B.8��1
C.1��5
D.5��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼʾ���Ӧ�����������������
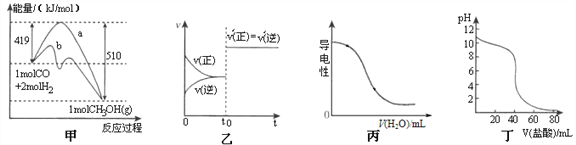
A. ͼ�ױ�ʾ��ҵ����CO�����״��ķ�ӦCO(g)+2H2(g)![]() CH3OH(g)���÷�Ӧ�ġ�H=��91.kJ��mol-1
CH3OH(g)���÷�Ӧ�ġ�H=��91.kJ��mol-1
B. ͼ�ұ�ʾ�Ѵ�ƽ���ij��Ӧ����t0ʱ�ı�ijһ������Ӧ������ʱ��仯����ı�����������Ǽ������
C. ͼ����ʾ��0.1 mol��L�C1�İ�ˮ��Һ����ˮʱ��Һ�ĵ����Ա仯
D. ͼ����ʾ����μӵ�0.1mol��L-1ij����Һ�õ��ĵζ����ߣ�����֪Ũ������ζ�δ֪Ũ�ȸü�ʱ���ѡȡ��̪��ָʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���1mol��CuSO4��5H2O��s������ˮ��ʹ��Һ�¶Ƚ��ͣ�����̱�ʾΪ��CuSO4��5H2O��s�� = Cu2+��aq��+SO42-��aq��+5 H2O��l�� ��ЧӦΪ��H1���� 1mol CuSO4��s������ˮ��ʹ��Һ�¶����ߣ�����̱�ʾΪ��CuSO4��s�� =Cu2+��aq��+SO42-��aq�� ��ЧӦΪ��H2��CuSO4��5H2O���ȷֽ�Ļ�ѧ����ʽΪ��CuSO4��5H2O��s�� ![]() CuSO4��s��+5H2O��l������ЧӦΪ��H3���������ж���ȷ������ ��
CuSO4��s��+5H2O��l������ЧӦΪ��H3���������ж���ȷ������ ��
A����H1����H3 B����H2����H3
C����H1+��H3 =��H2 D����H1+��H2 ����H3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������ȷ����( )
A. 1��5�����ױ� B. ����ױ� C. �ڶ��ױ� D. 1��4�����ױ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��Һ��ʯ����Һ�Ժ�ɫ�����и������������ڸ���Һ�д����������
A.H����NO3����Ca2����Cl��
B��Cu2����SO42����HCO3����Na��
C��Fe2����NO3����OH����Ba2��
D��MnO4����SO42����NO3����CO32��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������֮���ת������ͼ��ʾ��

����B��������������ά�����Ӧ�ĵ��ʵĽṹ����ʯ�Ľṹ�����Ƶġ�
��1�����Ʋ⣺A______��F___________(д��ѧʽ)
��2����д����Ӧ�ܵ����ӷ���ʽ��__________��
��3��д����Ӧ�ٵĻ�ѧ����ʽ____________��
��4���ڷ�Ӧ���У�̼��������______��������1molAʱ��ת�Ƶ��ӵ���ĿΪ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ�м����������ʣ���ˮ�ĵ��ƽ�ⲻ����Ӱ�����
A. NaHSO4 B. CH3COOK C. KAl(SO4)2 D. NaI
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com