
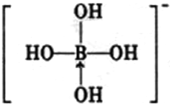
 +H+£¬µ¼ÖĀČÜŅŗ³ŹĖįŠŌ£®
+H+£¬µ¼ÖĀČÜŅŗ³ŹĖįŠŌ£® £¬½šŹōŠŌŌ½ĒæµÄŌŖĖŲĘä¼īµÄ¼īŠŌŌ½Ē棬øĘµÄ½šŹōŠŌ“óÓŚĆ¾£¬ĖłŅŌĒāŃõ»ÆøĘµÄ¼īŠŌ“óÓŚĒāŃõ»ÆĆ¾£¬¹Ź“š°øĪŖ£ŗ
£¬½šŹōŠŌŌ½ĒæµÄŌŖĖŲĘä¼īµÄ¼īŠŌŌ½Ē棬øĘµÄ½šŹōŠŌ“óÓŚĆ¾£¬ĖłŅŌĒāŃõ»ÆøĘµÄ¼īŠŌ“óÓŚĒāŃõ»ÆĆ¾£¬¹Ź“š°øĪŖ£ŗ £»Čõ£»
£»Čõ£»

 Ó¦ÓĆĢāµć²¦ĻµĮŠ“š°ø
Ó¦ÓĆĢāµć²¦ĻµĮŠ“š°ø דŌŖ¼°µŚĻµĮŠ“š°ø
דŌŖ¼°µŚĻµĮŠ“š°ø Ķ¬²½°ĀŹżĻµĮŠ“š°ø
Ķ¬²½°ĀŹżĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| »Æѧ¼ü | N”ŌN | H-O | N-H | O=O |
| ¼üÄÜ/kJ?mol-1 | 945 | 463 | 391 | 498 |
 2NH3£Øg£©+
2NH3£Øg£©+| 3 |
| 2 |
 2NH3£Øg£©+
2NH3£Øg£©+| 3 |
| 2 |
| T/”ę | 30 | 40 | 50 |
| Éś³ÉNH3Įæ/£Ø10-6mol/L£© | 4.8 | 5.9 | 6.0 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ½ĖÕŹ”øßČż°ŁŠ£“óĮŖæ¼Ņ»Ä£æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
£Ø15·Ö£©ĀČĖį¼ŲŹĒĪŽ»śŃĪ¹¤ŅµµÄÖŲŅŖ²śĘ·Ö®Ņ»£¬æÉĶعż·“Ó¦£ŗNaC1O3+KC1
KC1O3”ż+NaC1ÖĘČ””£
£Ø1£©ŹµŃéŹŅÖĘČ”ĀČĖįÄĘæÉĶعż·“Ó¦£ŗ3C12+6NaOH 5NaC1+NaC1O3+3H2O£¬½ńŌŚ”Ŗ5”ęµÄNaOHČÜŅŗÖŠĶØČėŹŹĮæC12£ØĘ½ŗā³£ŹżK=1.09”Į1012£©£¬“ĖŹ±C12µÄŃõ»Æ²śĪļÖ÷ŅŖŹĒ £»½«ČÜŅŗ¼ÓČČ£¬ČÜŅŗÖŠÖ÷ŅŖĄė×ÓÅضČĖęĪĀ¶ČµÄ±ä»ÆČēÓŅĶ¼ĖłŹ¾£¬Ķ¼ÖŠA”¢B”¢CŅĄ“Ī±ķŹ¾µÄĄė×ÓŹĒ ”£
5NaC1+NaC1O3+3H2O£¬½ńŌŚ”Ŗ5”ęµÄNaOHČÜŅŗÖŠĶØČėŹŹĮæC12£ØĘ½ŗā³£ŹżK=1.09”Į1012£©£¬“ĖŹ±C12µÄŃõ»Æ²śĪļÖ÷ŅŖŹĒ £»½«ČÜŅŗ¼ÓČČ£¬ČÜŅŗÖŠÖ÷ŅŖĄė×ÓÅضČĖęĪĀ¶ČµÄ±ä»ÆČēÓŅĶ¼ĖłŹ¾£¬Ķ¼ÖŠA”¢B”¢CŅĄ“Ī±ķŹ¾µÄĄė×ÓŹĒ ”£

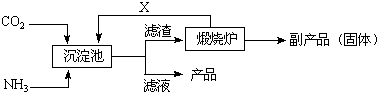
£Ø2£©¹¤ŅµÉĻÖĘČ”ĀČĖįÄĘ²ÉÓĆŌŚČČµÄŹÆ»ŅČéĶØČėĀČĘų£¬Č»
ŗó½į¾§³żČ„ĀČ»ÆøĘŗó£¬ŌŁ¼ÓČėŅ»ÖÖÄĘŃĪ£¬ŗĻŹŹµÄÄĘŃĪŹĒ
ӣ
£Ø3£©±±ĆĄ”¢Å·ÖŽ¹ś¼ŅÉś²śĀČĖįÄĘÓƶž¼¶¾«ÖĘŃĪĖ®”£²ÉÓĆ
ĪŽøōĤµē½ā·Ø»ńµĆ£¬Éś²ś¹ż³ĢÖŠÉę¼°µÄÖ÷ŅŖµÄ»Æѧ·“Ó¦Ź½ČēĻĀ£ŗ
×Ü·“Ó¦Ź½£ŗNaC1+3H2O NaC1O3+3H2”ü
NaC1O3+3H2ӟ
Ńō¼«£ŗ2C1”Ŗ”Ŗ2e”Ŗ
C12”üŅõ¼«£ŗ2H2O+2e”Ŗ
H2”ü+2OH”Ŗ
ŅŗĻą·“Ó¦£ŗC12+H2O HC1O+H++C1”Ŗ
HC1O
HC1O+H++C1”Ŗ
HC1O H++C1O”Ŗ
H++C1O”Ŗ
2HC1O+CO”Ŗ C1O3”Ŗ+2C1”Ŗ+2H+
¢Ł
¾«ÖĘŹ³ŃĪĖ®Ź±£¬ŅŖ³żČ„ĘäÖŠµÄCa2+”¢Mg2+¼°SO42”Ŗ²¢µĆµ½ÖŠŠŌČÜŅŗ£¬ŅĄ“Ī¼ÓČėµÄ»ÆѧŹŌ¼Į
¢Ś ŹĒ ”¢ ”¢ £»¹żĀĖ£¬ĀĖŅŗÖŠŌŁ¼ÓČėŹŹĮæµÄĻ”ŃĪĖį£¬µĆŅ»¼¶¾«ÖĘŃĪĖ®ŌŁ¾Ąė×Ó½»»»“¦Ąķ»ņĤ“¦ĄķµĆµ½¶ž¼¶¾«ÖĘŃĪĖ®”£
¢Śµē½āŹ±£¬±ŲŠėŌŚŹ³ŃĪĖ®ÖŠ¼ÓČėNa2Cr2O2£¬ĘäÄæµÄŹĒ·ĄÖ¹ £ØĢīĄė×Ó·ūŗÅ£©µē½ā¹ż³ĢÖŠŌŚŅõ¼«ÉĻ·Åµē”£
£Ø4£©ČōNaC1O2ÓėKC1µÄ»ģŗĻČÜŅŗÖŠNaC1O3ÓėKC1µÄÖŹĮæ·ÖŹż·Ö±šĪŖ0.290ŗĶ0.203£ØĻą¹ŲĪļÖŹµÄČܽā¶ČĒśĻßČēÓŅĶ¼£©”£“Ó»ģŗĻČÜŅŗÖŠ»ńµĆ½Ļ¶ąKC1O3¾§ĢåµÄŹµŃé²Ł×÷ŅĄ“Ī
ĪŖ £ØĢī²Ł×÷Ćū³Ę£©”¢øÉŌļ”£
£Ø5£©ŃłĘ·ÖŠC1O3”ŖµÄŗ¬ĮææÉÓƵĪ¶Ø·Ø½ųŠŠ²ā¶Ø£¬ŹµŃé²½Öč
ČēĻĀ£ŗ
²½Öč1£ŗ×¼Č·³ĘȔѳʷag£ØŌ¼2.20g£©£¬¾Čܽā”¢¶ØČŻµČ²½Öč×¼Č·ÅäÖĘ1000mLČÜŅŗ”£
²½Öč2£ŗ“ÓÉĻŹöČŻĮæĘæÖŠČ”³ö10.00mLӌ׶ŠĪĘæÖŠ£¬×¼Č·¼ÓČė25mL1000mol/L(NH4)2Fe£ØSO4£©2”£ČÜŅŗ£Ø¹żĮ棩£¬¼ÓČė75mLĮņĖįŗĶĮ×ĖįÅä³ÉµÄ»ģĖį£¬¾²ÖĆ10min”£
²½Öč3£ŗŌŁŌŚ×¶ŠĪĘæÖŠ¼ÓČė100mLÕōĮóĖ®¼°Ä³ÖÖŃõ»Æ»¹Ō·“Ó¦ÖøŹ¾¼Į£¬ÓĆ0.200mol/LK2Cr2O2±ź×¼ČÜŅŗµĪ¶ØÖĮÖÕµć”£
²½Öč4£ŗ ”£
²½Öč5£ŗŹż¾Ż“¦ĄķÓė¼ĘĖć”£
¢Ł²½Öč2£¬¾²ÖĆ10minµÄÄæµÄŹĒ ”£
¢Ś²½Öč3ÖŠK2Cr2O2±ź×¼ČÜŅŗÓ¦Ź¢·ÅŌŚ ÖŠ£ØĢīŅĒĘ÷Ćū³Ę£©”£
¢ŪĪŖĮĖČ·¶Øѳʷ֊C1O2”ŖµÄÖŹĮæ·ÖŹż£¬²½Öč4µÄ²Ł×÷ÄŚČŻŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
£Ø15·Ö£©ĀČĖį¼ŲŹĒĪŽ»śŃĪ¹¤ŅµµÄÖŲŅŖ²śĘ·Ö®Ņ»£¬æÉĶعż·“Ó¦£ŗNaC1O3+KC1 KC1O3”ż+NaC1ÖĘČ””£
£Ø1£©ŹµŃéŹŅÖĘČ”ĀČĖįÄĘæÉĶعż·“Ó¦£ŗ3C12+6NaOH![]() 5NaC1+NaC1O3+3H2O£¬½ńŌŚ”Ŗ5”ęµÄNaOHČÜŅŗÖŠĶØČėŹŹĮæC12£ØĘ½ŗā³£ŹżK=1.09”Į1012£©£¬“ĖŹ±C12µÄŃõ»Æ²śĪļÖ÷ŅŖŹĒ £»½«ČÜŅŗ¼ÓČČ£¬ČÜŅŗÖŠÖ÷ŅŖĄė×ÓÅضČĖęĪĀ¶ČµÄ±ä»ÆČēÓŅĶ¼ĖłŹ¾£¬Ķ¼ÖŠA”¢B”¢CŅĄ“Ī±ķŹ¾µÄĄė×ÓŹĒ ”£
5NaC1+NaC1O3+3H2O£¬½ńŌŚ”Ŗ5”ęµÄNaOHČÜŅŗÖŠĶØČėŹŹĮæC12£ØĘ½ŗā³£ŹżK=1.09”Į1012£©£¬“ĖŹ±C12µÄŃõ»Æ²śĪļÖ÷ŅŖŹĒ £»½«ČÜŅŗ¼ÓČČ£¬ČÜŅŗÖŠÖ÷ŅŖĄė×ÓÅضČĖęĪĀ¶ČµÄ±ä»ÆČēÓŅĶ¼ĖłŹ¾£¬Ķ¼ÖŠA”¢B”¢CŅĄ“Ī±ķŹ¾µÄĄė×ÓŹĒ ”£
£Ø2£©¹¤ŅµÉĻÖĘČ”ĀČĖįÄĘ²ÉÓĆŌŚČČµÄŹÆ»ŅČéĶØČėĀČĘų£¬Č»
ŗó½į¾§³żČ„ĀČ»ÆøĘŗó£¬ŌŁ¼ÓČėŅ»ÖÖÄĘŃĪ£¬ŗĻŹŹµÄÄĘŃĪŹĒ
ӣ
£Ø3£©±±ĆĄ”¢Å·ÖŽ¹ś¼ŅÉś²śĀČĖįÄĘÓƶž¼¶¾«ÖĘŃĪĖ®”£²ÉÓĆ
ĪŽøōĤµē½ā·Ø»ńµĆ£¬Éś²ś¹ż³ĢÖŠÉę¼°µÄÖ÷ŅŖµÄ»Æѧ·“Ó¦Ź½ČēĻĀ£ŗ
×Ü·“Ó¦Ź½£ŗNaC1+3H2O![]() NaC1O3+3H2”ü
NaC1O3+3H2ӟ
Ńō¼«£ŗ2C1”Ŗ”Ŗ2e”Ŗ C12”üŅõ¼«£ŗ2H2O+2e”Ŗ H2”ü+2OH”Ŗ
ŅŗĻą·“Ó¦£ŗC12+H2O![]() HC1O+H++C1”Ŗ HC1O
HC1O+H++C1”Ŗ HC1O![]() H++C1O”Ŗ
H++C1O”Ŗ
2HC1O+CO”Ŗ C1O3”Ŗ+2C1”Ŗ+2H+
¢Ł ¾«ÖĘŹ³ŃĪĖ®Ź±£¬ŅŖ³żČ„ĘäÖŠµÄCa2+”¢Mg2+¼°SO42”Ŗ²¢µĆµ½ÖŠŠŌČÜŅŗ£¬ŅĄ“Ī¼ÓČėµÄ»ÆѧŹŌ¼Į
¢Ś ŹĒ ”¢ ”¢ £»¹żĀĖ£¬ĀĖŅŗÖŠŌŁ¼ÓČėŹŹĮæµÄĻ”ŃĪĖį£¬µĆŅ»¼¶¾«ÖĘŃĪĖ®ŌŁ¾Ąė×Ó½»»»“¦Ąķ»ņĤ“¦ĄķµĆµ½¶ž¼¶¾«ÖĘŃĪĖ®”£
¢Śµē½āŹ±£¬±ŲŠėŌŚŹ³ŃĪĖ®ÖŠ¼ÓČėNa2Cr2O2£¬ĘäÄæµÄŹĒ·ĄÖ¹ £ØĢīĄė×Ó·ūŗÅ£©µē½ā¹ż³ĢÖŠŌŚŅõ¼«ÉĻ·Åµē”£
£Ø4£©ČōNaC1O2ÓėKC1µÄ»ģŗĻČÜŅŗÖŠNaC1O3ÓėKC1µÄÖŹĮæ·ÖŹż·Ö±šĪŖ0.290ŗĶ0.203£ØĻą¹ŲĪļÖŹµÄČܽā¶ČĒśĻßČēÓŅĶ¼£©”£“Ó»ģŗĻČÜŅŗÖŠ»ńµĆ½Ļ¶ąKC1O3¾§ĢåµÄŹµŃé²Ł×÷ŅĄ“Ī
ĪŖ £ØĢī²Ł×÷Ćū³Ę£©”¢øÉŌļ”£
£Ø5£©ŃłĘ·ÖŠC1O3”ŖµÄŗ¬ĮææÉÓƵĪ¶Ø·Ø½ųŠŠ²ā¶Ø£¬ŹµŃé²½Öč
ČēĻĀ£ŗ
²½Öč1£ŗ×¼Č·³ĘȔѳʷag£ØŌ¼2.20g£©£¬¾Čܽā”¢¶ØČŻµČ²½Öč×¼Č·ÅäÖĘ1000mLČÜŅŗ”£
²½Öč2£ŗ“ÓÉĻŹöČŻĮæĘæÖŠČ”³ö10.00mLӌ׶ŠĪĘæÖŠ£¬×¼Č·¼ÓČė25mL1000mol/L(NH4)2Fe£ØSO4£©2”£ČÜŅŗ£Ø¹żĮ棩£¬¼ÓČė75mLĮņĖįŗĶĮ×ĖįÅä³ÉµÄ»ģĖį£¬¾²ÖĆ10min”£
²½Öč3£ŗŌŁŌŚ×¶ŠĪĘæÖŠ¼ÓČė100mLÕōĮóĖ®¼°Ä³ÖÖŃõ»Æ»¹Ō·“Ó¦ÖøŹ¾¼Į£¬ÓĆ0.200mol/LK2Cr2O2±ź×¼ČÜŅŗµĪ¶ØÖĮÖÕµć”£
²½Öč4£ŗ ”£
²½Öč5£ŗŹż¾Ż“¦ĄķÓė¼ĘĖć”£
¢Ł²½Öč2£¬¾²ÖĆ10minµÄÄæµÄŹĒ ”£
¢Ś²½Öč3ÖŠK2Cr2O2±ź×¼ČÜŅŗÓ¦Ź¢·ÅŌŚ ÖŠ£ØĢīŅĒĘ÷Ćū³Ę£©”£
¢ŪĪŖĮĖČ·¶Øѳʷ֊C1O2”ŖµÄÖŹĮæ·ÖŹż£¬²½Öč4µÄ²Ł×÷ÄŚČŻŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģ½ĖÕŹ”øßČż°ŁŠ£“óĮŖæ¼Ņ»Ä£æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
£Ø15·Ö£©ĀČĖį¼ŲŹĒĪŽ»śŃĪ¹¤ŅµµÄÖŲŅŖ²śĘ·Ö®Ņ»£¬æÉĶعż·“Ó¦£ŗNaC1O3+KC1 KC1O3”ż+NaC1ÖĘČ””£
£Ø1£©ŹµŃéŹŅÖĘČ”ĀČĖįÄĘæÉĶعż·“Ó¦£ŗ3C12+6NaOH 5NaC1+NaC1O3+3H2O£¬½ńŌŚ”Ŗ5”ęµÄNaOHČÜŅŗÖŠĶØČėŹŹĮæC12£ØĘ½ŗā³£ŹżK=1.09”Į1012£©£¬“ĖŹ±C12µÄŃõ»Æ²śĪļÖ÷ŅŖŹĒ £»½«ČÜŅŗ¼ÓČČ£¬ČÜŅŗÖŠÖ÷ŅŖĄė×ÓÅضČĖęĪĀ¶ČµÄ±ä»ÆČēÓŅĶ¼ĖłŹ¾£¬Ķ¼ÖŠA”¢B”¢CŅĄ“Ī±ķŹ¾µÄĄė×ÓŹĒ ”£
5NaC1+NaC1O3+3H2O£¬½ńŌŚ”Ŗ5”ęµÄNaOHČÜŅŗÖŠĶØČėŹŹĮæC12£ØĘ½ŗā³£ŹżK=1.09”Į1012£©£¬“ĖŹ±C12µÄŃõ»Æ²śĪļÖ÷ŅŖŹĒ £»½«ČÜŅŗ¼ÓČČ£¬ČÜŅŗÖŠÖ÷ŅŖĄė×ÓÅضČĖęĪĀ¶ČµÄ±ä»ÆČēÓŅĶ¼ĖłŹ¾£¬Ķ¼ÖŠA”¢B”¢CŅĄ“Ī±ķŹ¾µÄĄė×ÓŹĒ ”£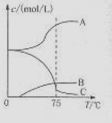
£Ø2£©¹¤ŅµÉĻÖĘČ”ĀČĖįÄĘ²ÉÓĆŌŚČČµÄŹÆ»ŅČéĶØČėĀČĘų£¬Č»
ŗó½į¾§³żČ„ĀČ»ÆøĘŗó£¬ŌŁ¼ÓČėŅ»ÖÖÄĘŃĪ£¬ŗĻŹŹµÄÄĘŃĪŹĒ
ӣ
£Ø3£©±±ĆĄ”¢Å·ÖŽ¹ś¼ŅÉś²śĀČĖįÄĘÓƶž¼¶¾«ÖĘŃĪĖ®”£²ÉÓĆ
ĪŽøōĤµē½ā·Ø»ńµĆ£¬Éś²ś¹ż³ĢÖŠÉę¼°µÄÖ÷ŅŖµÄ»Æѧ·“Ó¦Ź½ČēĻĀ£ŗ
×Ü·“Ó¦Ź½£ŗNaC1+3H2O NaC1O3+3H2”ü
NaC1O3+3H2ӟ
Ńō¼«£ŗ2C1”Ŗ”Ŗ2e”Ŗ C12”üŅõ¼«£ŗ2H2O+2e”Ŗ H2”ü+2OH”Ŗ
ŅŗĻą·“Ó¦£ŗC12+H2O HC1O+H++C1”Ŗ HC1O
HC1O+H++C1”Ŗ HC1O H++C1O”Ŗ
H++C1O”Ŗ
2HC1O+CO”Ŗ C1O3”Ŗ+2C1”Ŗ+2H+
¾«ÖĘŹ³ŃĪĖ®Ź±£¬ŅŖ³żČ„ĘäÖŠµÄCa2+”¢Mg2+¼°SO42”Ŗ²¢µĆµ½ÖŠŠŌČÜŅŗ£¬ŅĄ“Ī¼ÓČėµÄ»ÆѧŹŌ¼Į
ŹĒ ”¢ ”¢ £»¹żĀĖ£¬ĀĖŅŗÖŠŌŁ¼ÓČėŹŹĮæµÄĻ”ŃĪĖį£¬µĆŅ»¼¶¾«ÖĘŃĪĖ®ŌŁ¾Ąė×Ó½»»»“¦Ąķ»ņĤ“¦ĄķµĆµ½¶ž¼¶¾«ÖĘŃĪĖ®”£
¢Śµē½āŹ±£¬±ŲŠėŌŚŹ³ŃĪĖ®ÖŠ¼ÓČėNa2Cr2O7£¬ĘäÄæµÄŹĒ·ĄÖ¹ £ØĢīĄė×Ó·ūŗÅ£©µē½ā¹ż³ĢÖŠŌŚŅõ¼«ÉĻ·Åµē”£
£Ø4£©ČōNaC1O3ÓėKC1µÄ»ģŗĻČÜŅŗÖŠNaC1O3ÓėKC1µÄÖŹĮæ·ÖŹż·Ö±šĪŖ0.290ŗĶ0.203£ØĻą¹ŲĪļÖŹµÄČܽā¶ČĒśĻßČēÓŅĶ¼£©”£“Ó»ģŗĻČÜŅŗÖŠ»ńµĆ½Ļ¶ąKC1O3¾§ĢåµÄŹµŃé²Ł×÷ŅĄ“Ī
ĪŖ £ØĢī²Ł×÷Ćū³Ę£©”¢øÉŌļ”£
£Ø5£©ŃłĘ·ÖŠC1O3”ŖµÄŗ¬ĮææÉÓƵĪ¶Ø·Ø½ųŠŠ²ā¶Ø£¬ŹµŃé²½Öč
ČēĻĀ£ŗ
²½Öč1£ŗ×¼Č·³ĘȔѳʷag£ØŌ¼2.20g£©£¬¾Čܽā”¢¶ØČŻµČ²½Öč×¼Č·ÅäÖĘ1000mLČÜŅŗ”£
²½Öč2£ŗ“ÓÉĻŹöČŻĮæĘæÖŠČ”³ö10.00mLӌ׶ŠĪĘæÖŠ£¬×¼Č·¼ÓČė25mL1000mol/L(NH4)2Fe£ØSO4£©2”£ČÜŅŗ£Ø¹żĮ棩£¬¼ÓČė75mLĮņĖįŗĶĮ×ĖįÅä³ÉµÄ»ģĖį£¬¾²ÖĆ10min”£
²½Öč3£ŗŌŁŌŚ×¶ŠĪĘæÖŠ¼ÓČė100mLÕōĮóĖ®¼°Ä³ÖÖŃõ»Æ»¹Ō·“Ó¦ÖøŹ¾¼Į£¬ÓĆ0.200mol/LK2Cr2O7±ź×¼ČÜŅŗµĪ¶ØÖĮÖÕµć”£
²½Öč4£ŗ ”£
²½Öč5£ŗŹż¾Ż“¦ĄķÓė¼ĘĖć”£
¢Ł²½Öč2£¬¾²ÖĆ10minµÄÄæµÄŹĒ ”£
¢Ś²½Öč3ÖŠK2Cr2O2±ź×¼ČÜŅŗÓ¦Ź¢·ÅŌŚ ÖŠ£ØĢīŅĒĘ÷Ćū³Ę£©”£
¢ŪĪŖĮĖČ·¶Øѳʷ֊C1O3”ŖµÄÖŹĮæ·ÖŹż£¬²½Öč4µÄ²Ł×÷ÄŚČŻŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com