
�������������г������л���֮һ������Ĺ�����Ϊ____________��д���ƣ�����ʳ������ˮ������Ҫ�ɷ���CaCO3����ůƿ��ˮ��������������е�ˮ����д���÷�Ӧ�Ļ�ѧ����ʽ��
 __________________________________________��
__________________________________________��
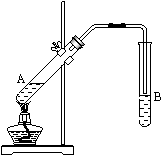
�����Թ�A�м���3mL�Ҵ���Ȼ������Թܱ���������2mLŨ�����2mL���ᣬ����ͼ��ʾ���Ӻ�װ�ý���ʵ�飬����ȡ����������
��1���Թ�B��ʢ�ŵ���Һ��__________________���������ǣ�___________________________________________________��
��2���Ҵ������ᷴӦ�Ļ�ѧ����ʽ�ǣ�___________________
_____________________����Ӧ������__________________��
��3��ʵ����ɺ��Թ�B��Һ���������IJ�����ˮ����״Һ�������������B�е�Һ��������Ҫ�õ��IJ���������Ҫ��_____________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �������������г������л���֮һ������Ĺ�����Ϊ
�������������г������л���֮һ������Ĺ�����Ϊ CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �������������г������л���֮һ������Ĺ�����Ϊ
�������������г������л���֮һ������Ĺ�����Ϊ CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���Ҵ������ᶼ�����Ʒ�Ӧ | B���Ҵ������ụΪͬ���칹�� | C���Ҵ������ᶼ����NaOH��Һ��Ӧ | D���Ҵ������ᶼ��ʹ��ɫʯ����Һ���ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�꽭��ʡ������ѧ��ѧҵˮƽ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�Ҵ��������������г����������л������˵����ȷ����
A���Ҵ������ᶼ�����Ʒ�Ӧ
B���Ҵ������ụΪͬ���칹��
C���Ҵ������ᶼ����NaOH��Һ��Ӧ
D���Ҵ������ᶼ��ʹ��ɫʯ����Һ���ɫ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com