
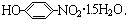 ��
�� ���ĵ���ƽ�ⳣ��K2����Դ�С
���ĵ���ƽ�ⳣ��K2����Դ�С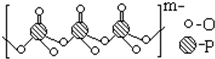


 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��KԽ�ﵽƽ��ʱ����Ӧ���еij̶�Խ�� |
| B��K�ı���ʽ��K=Cp��C��?Cq��D��/Cm��A��?Cn��B�� |
| C����Qc��K����Ӧ���淴Ӧ������� |
| D�������淴Ӧ��ƽ�ⳣ����ֵ��ͬ�������෴ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
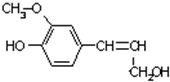 ľ�������ƽ���ֽ��ҵ�ĸ��������������������ѧ�ص㣬ľ���ص�һ�ֵ���ṹ��ʽ��ͼ��ʾ������˵��������ǣ�������
ľ�������ƽ���ֽ��ҵ�ĸ��������������������ѧ�ص㣬ľ���ص�һ�ֵ���ṹ��ʽ��ͼ��ʾ������˵��������ǣ�������| A�������ʵķ���ʽ��C10H12O3�����ڷ����� |
| B�������ʿ�����FeCl3��Һ������ɫ��Ӧ��Ҳ�ܷ���������Ӧ |
| C��1mol���������������4mol H2 |
| D��1mol����������ˮ������Ӧ��������4mol Br2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������Aԭ�ӻ�̬ʱ���������������ڲ����������2����Bԭ�ӻ�̬ʱs��������P��������ȣ�C��Ԫ�����ڱ��ĸ�Ԫ���е縺�����D�Ļ�̬ԭ�Ӻ�����6���ܼ���ȫ���������ӣ�Eԭ�ӻ�̬ʱδ�ɶԵ�������ͬ����Ԫ�������ģ�
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������Aԭ�ӻ�̬ʱ���������������ڲ����������2����Bԭ�ӻ�̬ʱs��������P��������ȣ�C��Ԫ�����ڱ��ĸ�Ԫ���е縺�����D�Ļ�̬ԭ�Ӻ�����6���ܼ���ȫ���������ӣ�Eԭ�ӻ�̬ʱδ�ɶԵ�������ͬ����Ԫ�������ģ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
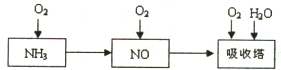 ��ҵ������Ϊԭ�ϣ�����Ͻ���Ϊ��������������������Ĺ������£�
��ҵ������Ϊԭ�ϣ�����Ͻ���Ϊ��������������������Ĺ������£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��100mL 3mol/L NaCl��Һ |
| B��200mL 2.5mol/L MgCl2��Һ |
| C��300mL 2mol/L AlCl3��Һ |
| D��400mL 1mol/L���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com