
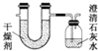
 ��֪ij��ȼ�Ϻ���̼�������Ԫ�أ�Ϊ�˲ⶨ����ȼ����̼��������Ԫ�ص������ȣ��ɽ���̬ȼ�Ϸ���������O2��ȼ�գ���������������ȫ��ͨ��ͼʾװ�ã��õ�������е�ʵ�����ݣ�������������ȫ�����գ���
��֪ij��ȼ�Ϻ���̼�������Ԫ�أ�Ϊ�˲ⶨ����ȼ����̼��������Ԫ�ص������ȣ��ɽ���̬ȼ�Ϸ���������O2��ȼ�գ���������������ȫ��ͨ��ͼʾװ�ã��õ�������е�ʵ�����ݣ�������������ȫ�����գ���| ʵ��ǰ | ʵ��� | |
| �������+U�ιܣ������� | 101.1g | 102.9g |
| ������ʯ��ˮ+���ƿ�������� | 312.0g | 314.2g |
| 1.8g |
| 18g/mol |
| 2.2g |
| 44g/mol |
| 0.05mol |
| 0.1mol��2 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���� | B���� | C���� | D���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��þԭ����1s22s22p63s2��1s22s22p63p2ʱ��ԭ���ͷ��������ɻ�̬ת���ɼ���̬ |
| B��ͬһԭ���У�2p��3p��4p�ܼ��Ĺ���������� |
| C������ԭ����һ�ܲ��s����������ͼ�������Σ�����İ뾶��С��ͬ |
| D��CH2O��ClO4-������ԭ���϶����й¶Ե��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������ӵ�1s�ܼ��ϵĵ���������ͬ |
| B�������ӵ�3p�ܼ��ϵĵ�����˵ľ�����ͬ |
| C�������ӵĵ��ӷ���ԾǨʱ�������Ĺ��ײ�ͬ |
| D�������Ӷ���8�����ȶ��ṹ����ѧ������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������COCl2�� |
| B�����Ȼ��ף�PCl5�� |
| C����������CF2Cl2�� |
| D����������BF3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
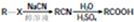
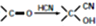
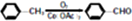
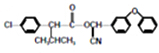 ��
��
 ע���ϳ�·�ߵ���д��ʽ��������ʾ������ͼ��
ע���ϳ�·�ߵ���д��ʽ��������ʾ������ͼ��
�鿴�𰸺ͽ���>>
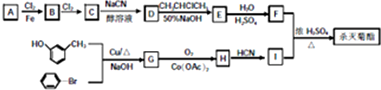
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
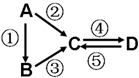 A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ����A�ǵ��ʣ�����֮�������µķ�Ӧ��ϵ��
A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ����A�ǵ��ʣ�����֮�������µķ�Ӧ��ϵ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
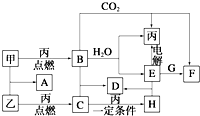 �ס��ҡ���Ϊ�������ʣ�A��B��C��D��E��F��G��H��Ϊ��ѧ��ѧ�г����Ļ��������B��G����ɫ��Ӧ��Ϊ��ɫ��C��ʹƷ����Һ��ɫ����һ�������£��������ת����ϵ��ͼ��ʾ����ش��������⣺
�ס��ҡ���Ϊ�������ʣ�A��B��C��D��E��F��G��H��Ϊ��ѧ��ѧ�г����Ļ��������B��G����ɫ��Ӧ��Ϊ��ɫ��C��ʹƷ����Һ��ɫ����һ�������£��������ת����ϵ��ͼ��ʾ����ش��������⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com