
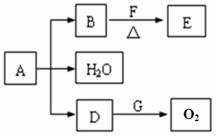
��֪A��B��D��E��F��GΪ��ѧ��ѧ�г����Ļ��������֮������ͼ��ʾ��ת����ϵ����Ӧ���������ֲ�������ȥ����AΪ��ɫ��ĩ����H��C��O��Cu����Ԫ�ء������£�DΪ��ɫ��ζ���壬BΪ��ɫ��ĩ��E���ӽṹ�к���ȩ����
��ش��������⣺
��1��D��G��Ӧ�Ļ�ѧ����ʽ ��
��2��F��һ�����еĹ��������� ��
��3��ij����С�����������ʵ��װ�ã�ͨ���ⶨװ�ü������Լ��������仯��̽��A�Ļ�ѧʽ��
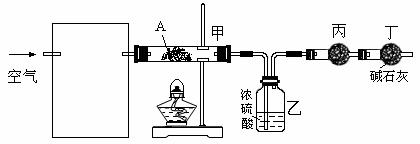
��Ϊʹ����ȷ�����貹��װ�ã������ڷ����ڻ��װ��ͼ��д���Լ����ơ�
����װ���й��������Ŀ���� ����װ����ҩƷ�������� ��ʵ��ʱ����ҩƷδ�����Ա仯��֤�� ��
������ж�A����ȫ�ֽ⣿ ��
��ʵ���ó��������ݣ�A���Ⱥ���ȫ�ֽ⣬������8.0 g��Ϊ6.0 g��װ��������0.9 g��д��A�Ļ�ѧʽ����ʾΪ��ʽ�Σ��� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��
��


| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?����ģ�⣩ԭ�����������IJ��ֶ���������Ԫ�ص�ԭ�Ӱ뾶�������±���
��2011?����ģ�⣩ԭ�����������IJ��ֶ���������Ԫ�ص�ԭ�Ӱ뾶�������±���| Ԫ�� | A | B | D | E | F | G | M | N | P |
| ԭ�Ӱ뾶/nm | 0.077 | 0.070 | 0.066 | 0.064 | 0.186 | 0.160 | 0.143 | 0.104 | 0.099 |


| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
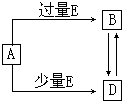 ��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����
��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com