
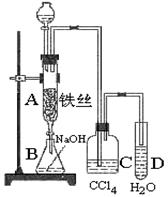
ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�� ���ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��С�
��1��д��A�з�Ӧ�Ļ�ѧ����ʽ
��ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ���� ��д���йط�Ӧ�Ļ�ѧ����ʽ ��
�� C��ʢ��CCl4�������� ��
��4����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤���÷�ӦΪȡ����Ӧ����һ����֤�ķ��������Թ�D�м��� �������� ��
��1��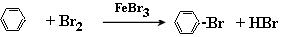
��2����ȥ�����屽�е���
Br2 +2 NaOH ��NaBr + NaBrO+ H2O �� 3Br2 + 6NaOH = 5NaBr + NaBrO3 + 3H2O
��3����ȥ�廯�������е�������
��4��ʯ����Һ����Һ���ɫ �����������𰸶����֣�
����������1��װ��A����ȡ�屽�ģ����Է���ʽΪ
 ��
��
��2���������ɵ��屽�к��е����壬������������������Һ��ȥ�����壬����ʽΪBr2 +2 NaOH ��NaBr + NaBrO+ H2O��
��3����Ϊ�������ӷ���Ϊ�˷�ֹ�����廯��ļ��飬��Ҫ��ȥ�塣�����������Ȼ�̼�У������������Ȼ�̼��ȥ�廯�������е���������
��4����Ϊ�廯������ˮ�����ԣ����Կ��������ָʾ�����м��顣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A(A�¶˻����ر�)�С�

(1)д��A�з�Ӧ�Ļ�ѧ����ʽ________________________________________________��
(2)�۲쵽A�е�������______________________________________________________��
(3)ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����______________��д���йصĻ�ѧ����ʽ_________________________________________________________��
(4)C��ʢ��CCl4��������_____________________________________________________��
(5)��֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м��룬������__________
________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)д��A�з�Ӧ�Ļ�ѧ����ʽ_________________________________��
(2)�۲쵽A�е�������______________________________________��
(3)ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����___________________��д���йصĻ�ѧ����ʽ________________��
(4)C��ʢ��CCl4��������______________________��
(5)��֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м���_____________��������______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)д��A�з�Ӧ�Ļ�ѧ����ʽ______________________________________________��
(2)�۲쵽A�е�������__________________________________________________��
(3)ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����_______________��д���йصĻ�ѧ����ʽ_____________________________________________________��
(4)C��ʢ��CCl4��������_______________________________________________��
(5)��֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м���___________________________��������_____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�� ���ٽ����Һ�������뷴Ӧ��A(A�¶˻����ر�)�С�
(1) д��A�з�Ӧ�Ļ�ѧ����ʽ______________________
(2) ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������
Ŀ����______ ___�� д���йصĻ�ѧ����ʽ��_______________ ____
(3) C��ʢ��CCl4�������� ��
(4)��֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ������
�Թ� D�м���AgNO3��Һ������������ɫ����������֤������һ����
֤�ķ��������Թ�D�м���______ ___�������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com