
(1)0.02mol/LµÄHCNČÜŅŗÓė0.02mol/LNaCNČÜŅŗµČĢå»ż»ģŗĻ£¬ŅŃÖŖøĆ»ģŗĻČÜŅŗÖŠ[![]() ]£¾[
]£¾[![]() ]£¬ÓĆ”°£¾”¢£¼”¢£½”±·ūŗÅĢīæÕ
]£¬ÓĆ”°£¾”¢£¼”¢£½”±·ūŗÅĢīæÕ
¢ŁČÜŅŗÖŠ[![]() ]________[
]________[![]() ] ¢Ś[HCN]________[
] ¢Ś[HCN]________[![]() ]
]
¢Ū[HCN]£«[![]() ]________0.02mol/L
]________0.02mol/L
(2)¢ŁĻņĆ÷·ÆČÜŅŗÖŠÖšµĪµĪČėĒāŃõ»Æ±µČÜŅŗÖĮĮņĖįøłĄė×ÓøÕŗĆ³ĮµķĶźČ«Ź±£¬ČÜŅŗµÄpH________7(Ģī£¾”¢£¼”¢£½)£¬Ąė×Ó·“Ó¦×Ü·½³ĢŹ½ĪŖ________£®
¢ŚĻņĆ÷·ÆČÜŅŗÖŠÖšµĪ¼ÓĒāŃõ»Æ±µČÜŅŗÖĮĀĮĄė×ÓøÕŗĆ³ĮµķĶźČ«Ź±£¬ČÜŅŗµÄpH________7(Ģī£¾”¢£¼”¢£½)Ąė×Ó·“Ó¦×Ü·½³ĢŹ½ĪŖ________£®

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ×鱚 | ¢ń | ¢ņ | ¢ó |
| ŃĪĖįµÄĢå»ż/mL | 50.00 | 50.00 | 50.00 |
| ¹ĢĢåAµÄÖŹĮæ/g | 3.800 | 6.200 | 7.200 |
| ĘųĢåµÄĢå»ż/mL | 896.0 | 1344 | 1344 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
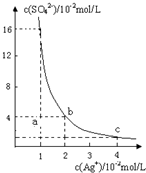 ijĪĀ¶ČŹ±£¬Ag2SO4ŌŚĖ®ČÜŅŗÖŠµÄ³ĮµķČܽāĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾£ŗ
ijĪĀ¶ČŹ±£¬Ag2SO4ŌŚĖ®ČÜŅŗÖŠµÄ³ĮµķČܽāĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

 3C£Øg£©”÷H=+200akJ/mol
3C£Øg£©”÷H=+200akJ/mol 3C£Øg£©”÷H=+200akJ/mol
3C£Øg£©”÷H=+200akJ/mol| 2c |
| 3 |
| c |
| 3 |
| 2c |
| 3 |
| c |
| 3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ŌŚŗ¬ÓŠČõµē½āÖŹµÄČÜŅŗÖŠ£¬ĶłĶłÓŠ¶ąøö»ÆŃ§Ę½ŗā¹²“ę£®
ŌŚŗ¬ÓŠČõµē½āÖŹµÄČÜŅŗÖŠ£¬ĶłĶłÓŠ¶ąøö»ÆŃ§Ę½ŗā¹²“ę£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com