
 ��1������VSEPRģ�ͣ�
��1������VSEPRģ�ͣ�| 1 |
| 8 |
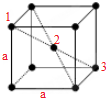 ��4r��2=a2+��
��4r��2=a2+��| 2 |
4
| ||
| 3 |
| ||
| �� |
| 1 |
| 2 |
| 1 |
| 8 |
4
| ||
| 3 |
4
| ||
| 3 |
| ||
| �� |
| 2M |
| NAd |
4
| ||
| 3 |
| 3 |
| ||||
| 3 |
| ||||


 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �¶�/�� | 25 | t1 | t2 |
| ˮ�����ӻ����� | 1��10-14 | KW | 1��10-12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
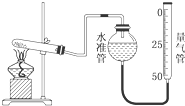 ijѧ�����ø�����طֽ��������ķ�Ӧ���ⶨ�����µ�����Ħ�������ʵ��װ����ͼ��ʾ������ʵ�鲽�裺��װ��ʵ��װ�ã��ڡ��۰������ĸ�����ط�ĩ���������Թ��У�ȷ�����Թܺ�����ط�ĩ������Ϊa g���ܼ��ȣ���ʼ��Ӧ��ֱ������һ���������壮��ֹͣ���ȣ������ռ�����������������ȷ�����ԹܺͲ����������Ϊb g�������ʵ���ҵ��¶ȣ��ش��������⣮
ijѧ�����ø�����طֽ��������ķ�Ӧ���ⶨ�����µ�����Ħ�������ʵ��װ����ͼ��ʾ������ʵ�鲽�裺��װ��ʵ��װ�ã��ڡ��۰������ĸ�����ط�ĩ���������Թ��У�ȷ�����Թܺ�����ط�ĩ������Ϊa g���ܼ��ȣ���ʼ��Ӧ��ֱ������һ���������壮��ֹͣ���ȣ������ռ�����������������ȷ�����ԹܺͲ����������Ϊb g�������ʵ���ҵ��¶ȣ��ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com