
��ѧ��ѧ��������A��һ�������·������·�Ӧ��A+B��E+F+H2O��δ��ƽ��
��1����AΪС�մ�FΪ���塣�÷�Ӧ�����ӷ���ʽΪ ��
��2����AΪ�Ϻ�ɫ�������ʣ�����F��������λ��ͬһ����Ķ�����Ԫ����ɡ���Ӧ�Ļ�ѧ����ʽΪ_____________________��
��3����A�Ǵ��������Ҫ�ɷ֣�B�����ᡣд����Ӧ�Ļ�ѧ����ʽΪ ��
��4����AΪ����ɫ���嵥�ʣ�F�ļ�����Һ���շ�����SO2�����ӷ���ʽΪ ��
��1��HCO3����H+��H2O��CO2����2�֣�
��2��Cu+2H2SO4=CuSO4+SO2+2H2O��2�֣�
��3��Fe3O4��8HCl��2FeCl3��FeCl2��4H2O(��Fe3O4��8H+��2Fe3+��Fe2+��4H2O)��2�֣�
��4��2OH��+ClO��+SO2��Cl��+SO42��+H2O��2�֣�
���������������1����AΪС�մ�������Ƴ�A����ķ�Ӧ����2����AΪCu������FΪSO 2��������Ƴ�Cu��Ũ����ķ�Ӧ����3����AΪ������ӦΪFe3O4��8HCl��2FeCl3��FeCl2��4H2O��4����AΪ����ɫ����Cl2�������д��Cl2��SO2�ķ�Ӧ�����õ��Ӻ͵���غ㡣
���㣺�����ƶϼ���ط���ʽ����д


 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D�����ֳ������ʣ����ӦԪ�ص�ԭ������������������B��D���ڳ������������Ϊ���������J��һ�ֺ�ɫ���壬I��Ũ��Һ���л�ԭ�ԣ�A��J����������֮�������µ�ת����ϵ�����ַ�Ӧ����ʡ�ԣ��� 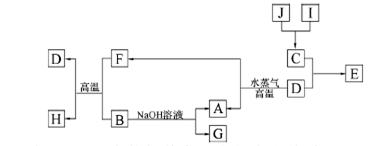
��1��BԪ�غ�CԪ�صļ����Ӱ뾶��С��ϵ�� �������ӷ��ű�ʾ����
��2������Ԫ����C�γɵĻ�����NC3����ˮ�в���ʹ��ɫʯ����ֽ���������壬д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3����E�ı�����Һ�����ˮ���γ���Һ�壬�ٽ���Һ��װ��U�ܣ�����U�ܵ����˲���缫����ֱͨ���磬�������˿ɹ۲쵽�������� ��
��4��������J�����ữ��H2O2����Һ�У�J�ܽ���������+2�����ӣ��÷�Ӧ�����ӷ���ʽ��
��
��5������0��1 mol G����Һ�еμ�5 mol/L ��������Һ���õ�����3��9 g �������������������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪A��B��C����ѧ��ѧ�ij������ʣ�������һ��������������ת����ϵ��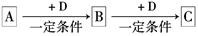
��1����A��ʹʪ��ĺ�ɫʯ����ֽ������CΪ����ɫ���塣��Aת��ΪB��Ӧ�Ļ�ѧ����ʽΪ ��
��2����D�ǽ�����C��Һ������ʱӦ��������D���������ǣ��ñ�Ҫ�����ֺ����ӷ���ʽ˵���� ��
��3����D��һ�ֳ������������壬A��һ��ǿ���������ˮ��Һ�е���������������Ӿ�����10�����ӣ�����C���ȷֽ�ɵù���B���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��4����DΪ�ȼҵ����Ҫ��Ʒ��B�������ԣ���Bת��ΪC�����ӷ���ʽΪ ��
��5����A��B��C��Ϊ�����D��һ�ֺ�ɫ��̬�ǽ������ʣ���Ԫ��D�����ڱ��е�λ
���� ��B���ӵĽṹʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D�������ʶ�����ͬ��Ԫ��������ͼ��ʾת����ϵ���Իش��������⣺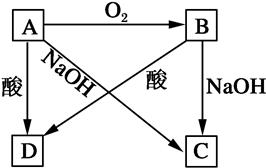
��1����A��һ�ֳ����ǽ���������һ�ְ뵼����ϡ�д�����з�Ӧ�ķ���ʽ��
��B��D�Ļ�ѧ����ʽ��__________________________________________________��
��A��C�����ӷ���ʽ��__________________________________________________��
��2����A��һ�ֳ�����������ʱ��Ϊǿ�ᡣд����A��D�����ӷ���ʽ��__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ�һ���ڱ��е�λ�ã��û�ѧ����ش��������⣺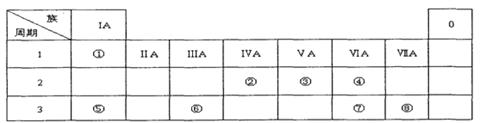
��1���뻭��Ԫ�آ�������ӽṹʾ��ͼ ��
��2���ܡ��ݡ��ߵ�ԭ�Ӱ뾶��С�����˳��Ϊ ��
��3���ݺ͢�����������Ӧˮ����ļ���ǿ��Ϊ > ��
��4���ܡ�������Ԫ�ص�ԭ�Ӱ�1:1��ɵij���������ĵ���ʽΪ ��
���ɱ��Т�һ���е�һ�ֻ���Ԫ���γɵij�������A��B��C�ɷ������·�Ӧ������������ȥ�����Իش�
��1����X��һ�ֳ������ɽ������ʣ���C��ˮ��Һ�еμ�AgNO3��Һ������������ϡHNO3�İ�ɫ�����������C��Һ�н������ӵķ����� ����֪��������Һ�иý��������ܱ�˫��ˮ������д���÷�Ӧ�����ӷ���ʽ ��
��2����A��B��CΪ����ͬһ����Ԫ�ص��������XΪǿ����ʣ�A��Һ��C��Һ��Ӧ����B����B�Ļ�ѧʽΪ ��д��A��C����Һ��Ӧ�����ӷ���ʽ____ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C����ѧ��ѧ�������������ʣ�����֮����ת����ϵ���£����ַ�Ӧ������������ȥ����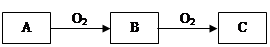
��1����A��һ�ֻ��ý�����C�ǵ���ɫ���壬���û�ѧ����ʽ��ʾ������C��һ����ҪӦ�� ��
��2����A��һ�ֻ�ɫ���ʹ��壬��B��C�Ļ�ѧ����ʽΪ ��
��3����A��һ�ֺ�ɫ���ʹ��壬д��B��һ����; ��
��4����C�Ǻ���ɫ���壬��A��ѧʽ����Ϊ ����д��C��ˮ��Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼΪ��ѧ�������ʼ��ת����ϵ�����мס��ҡ�������Ϊ���ʣ�����Ϊ�������������Ԫ������������������������C������Ϊ��ɫҺ�壬B��ɫ��ӦΪ��ɫ��һЩ����Һ�н��еķ�Ӧ��Һ�е�H2O�����ɵ�H2O��ʡ�ԡ�
�ش��������⣺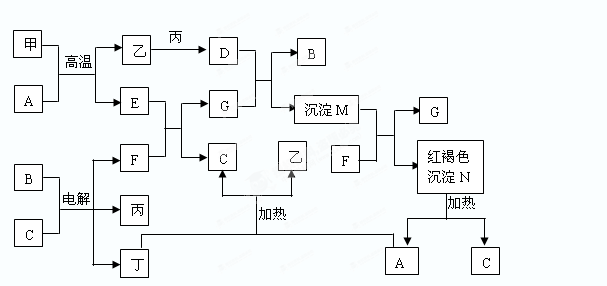
��1������������Ԫ�������ڱ��е�λ��______________________��
��2��������F�ĵ���ʽ________________________��
��3��D��G��Ӧ�����ӷ���ʽ___________________________________��
��4����A��Ӧ�Ļ�ѧ����ʽ___________________________________�����෴Ӧһ�㱻����________��Ӧ���÷�Ӧ����ҪӦ��_________________��(2��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ��ѧ��������A��һ�������·������·�Ӧ��A+B��E+F+H2O��δ��ƽ��
��1����AΪС�մ�FΪ���塣�÷�Ӧ�����ӷ���ʽΪ ��
��2����AΪ�Ϻ�ɫ�������ʣ�����F��������λ��ͬһ����Ķ�����Ԫ����ɡ���E�Ļ�ѧʽΪ________��
��3����A�Ǵ��������Ҫ�ɷ֣�B�����ᡣд����Ӧ�Ļ�ѧ����ʽΪ ��
��4����AΪ����ɫ���嵥�ʣ�F�ļ�����Һ���շ�����SO2�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������AΪһ�ֳ�������Ԫ����һ�ֳ����ǽ���Ԫ����ɵĻ�����������������ӵĸ�����Ϊ2:3��KΪ������̬�ǽ������ʣ�J��NΪ������̬���ʣ�����Ϊ���������I��F�ڳ�����ΪҺ̬��C��DΪ�̼������壬H��ɫ��ζ���壬BΪ��ɫ��״����,LΪ�ȼҵ�еij�����Ʒ��F��Ũ��Һ��K���ȿ�����D��H��(����������δ���)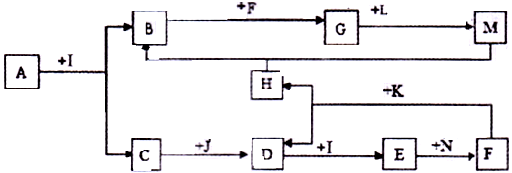
(1)H�ĽṹʽΪ: ��B�Ļ�ѧʽΪ: ��
(2)д�����б仯�Ļ�ѧ����ʽ:
A+I ��B+C�� ��
F��Ũ��Һ��K���ȿ�����D��H�� ��
(3)д�����б仯�����ӷ���ʽ:
Nͨ��E��ˮ��Һ: ��
M��ˮ��Һ��ͨ�˹�����H: ��
(4)��A��K��������������������ʵ���_ �����ڷǵ���ʵ��� (�ñ�Żش�)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com