
����������ʳ�����������ӣ�ũҵ�Ի�ѧ���ϵ�����ԽԽ��������������һ�ֻ��ʡ����ʵ������ͺ���ʩ����ũҵ�����������ش����á��ϳɰ���������������ʾ��ͼ���£�

��1��Ŀǰ����ҵ��������ý����������20��50 MPa��450�������õ�������������
�н��кϳɰ�������X�����ȥ�������� ��
��2���ں����Ƽ�У��ӳ������еõ���ĸҺҪͨ�˰��������²����� ���ѧʽ����Ŀ���� ��
��3���������еĻ�ѧ����ʽ�� ��
��4����ҵ�Ͻ����������������ᡣ�����������У����������ڰ��Ĵ�������Ӧ������� ���� �����Ṥҵβ���Ի�����Ⱦ���أ����ڴ����������ü��齫���е�һ��������ԭΪ�Կ�������Ⱦ�����ʣ��䷴Ӧ�Ļ�ѧ����ʽΪ ��
��1����2�֣��ϳ�����1�֣���H2��N2��1�֣�
��2��(2��)NaCl ��1�֣���ʹ�Ȼ����ĸҺ�нᾧ������1�֣�
��3����2�֣�NH3 + CO2 + H2O +NaCl = NH4Cl + NaHCO3��
��4����2�֣��ӿ췴Ӧ����ͬʱ����������������һ��������1�֣���
CH4 + 4NO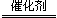 2N2 + CO2 + 2H2O ��1�֣�
2N2 + CO2 + 2H2O ��1�֣�
��������
�����������1�������������ķ�Ӧ�ںϳ����н��У���Ϊ�ϳɰ���ӦΪ���淴Ӧ����Ӧ�����к���һ�����ĵĵ��������� �����������Ϊԭ�Ͻ���ѭ�����á�
��2�������Ȼ��ƣ�����ͬ����ЧӦ�������Ȼ�淋��ܽ�ȣ�ʹ�Ȼ�什ᾧ������
��3����������ͼ����Ŀ������Ϣ����֪��Ӧ����NH3 ��CO2 ��H2O��NaCl������ΪNH4Cl �� NaHCO3��������д����ѧ����ʽ��
��4������������Ϊ�ӿ컯ѧ��Ӧ���ʣ�ͬʱ����NO�����ݻ��ϼ۵ı仯�����齫���е�һ��������ԭΪ�Կ�������Ⱦ�����ʣ�����N2��CO2��H2O ����ƽ�ɵû�ѧ����ʽ��
���㣺���⿼�黯ѧ�������̡���ѧ����ʽ����д�����������á�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ�����а�У��һ��ѧ����ĩ������ѧ�Ծ����������� ���ͣ������
��5�֣�����������ʳ��������������ũҵ�Ի�ѧ���ϵ�������Խ��Խ�����е���������������һ�ֻ��ʡ������ĺϳ�Ϊ���ʵ�������ҵ�춨�˻�������ԭ��Ϊ��N2��3H2 2NH3
2NH3
��1����N2��3H2 2NH3�ķ�Ӧ�У�һ��ʱ���NH3��Ũ��������0.9mol��L��1����N2��ʾ�䷴Ӧ����Ϊ0.15 mol��L��1��s��1������������ʱ��Ϊ ��
2NH3�ķ�Ӧ�У�һ��ʱ���NH3��Ũ��������0.9mol��L��1����N2��ʾ�䷴Ӧ����Ϊ0.15 mol��L��1��s��1������������ʱ��Ϊ ��
A��2 s B��3 s C��4 s D��6 s
��2������4���������ڲ�ͬ�����²�õĺϳɰ���Ӧ�����ʣ����з�Ӧ������ ��
A��v(H2)��0.1 mol��L��1��min��1 B��v(N2)��0.1 mol��L��1��min��1
C��v(NH3)��0.15 mol��L��1��min��1 D��v(N2)��0.002mol��L��1��min��1
��3����һ�����ȡ��ݻ�������ܱ������з������淴Ӧ��N2(g)��3H2(g) 2NH3(g) ��H��0�����и�����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬���� ��
2NH3(g) ��H��0�����и�����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬���� ��
A�������������ܶȱ��ֲ���
B���������¶Ȳ��ٱ仯
C������1mol N��N����ͬʱ������6 mol N��H��
D����Ӧ����N2��H2�����NH3������֮��1�U3�U2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ɹŰ���һ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��6�֣�����������ʳ��������������ũҵ�Ի�ѧ���ϵ�������Խ��Խ�����е���������������һ�ֻ��ʡ������ĺϳ�Ϊ���ʵ�������ҵ�춨�˻�������ԭ��Ϊ��N2��3H2 2NH3
2NH3
��1����N2��3H2 2NH3�ķ�Ӧ�У�һ��ʱ���NH3��Ũ��������0.9mol��L��1����N2��ʾ�䷴Ӧ����Ϊ0.15 mol��L��1��s��1������������ʱ��Ϊ ��
2NH3�ķ�Ӧ�У�һ��ʱ���NH3��Ũ��������0.9mol��L��1����N2��ʾ�䷴Ӧ����Ϊ0.15 mol��L��1��s��1������������ʱ��Ϊ ��
A��2 s B��3 s C��4 s D��6 s
��2������4���������ڲ�ͬ�����²�õĺϳɰ���Ӧ�����ʣ����з�Ӧ������ ��
A��v(H2)��0.1 mol��L��1��min��1 B��v(N2)��0.1 mol��L��1��min��1
C��v(NH3)��0.15 mol��L��1��min�� D��v(N2)��0.002mol��L��1��min��1
��3����һ�����ȡ��ݻ�������ܱ������з������淴Ӧ��
N2(g)��3H2(g) 2NH3(g) ��H��0�����и�����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬���� ��
2NH3(g) ��H��0�����и�����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬���� ��
A�������������ܶȱ��ֲ���
B���������¶Ȳ��ٱ仯
C������1mol N��N����ͬʱ������6 mol N��H��
D����Ӧ����N2��H2�����NH3������֮��1�U3�U2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭ʡ�����а�У��һ��ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ������
��5�֣�����������ʳ��������������ũҵ�Ի�ѧ���ϵ�������Խ��Խ�����е���������������һ�ֻ��ʡ������ĺϳ�Ϊ���ʵ�������ҵ�춨�˻�������ԭ��Ϊ��N2��3H2 2NH3
2NH3
��1����N2��3H2 2NH3�ķ�Ӧ�У�һ��ʱ���NH3��Ũ��������0.9mol��L��1����N2��ʾ�䷴Ӧ����Ϊ0.15 mol��L��1��s��1������������ʱ��Ϊ
��
2NH3�ķ�Ӧ�У�һ��ʱ���NH3��Ũ��������0.9mol��L��1����N2��ʾ�䷴Ӧ����Ϊ0.15 mol��L��1��s��1������������ʱ��Ϊ
��
A��2 s B��3 s C��4 s D��6 s
��2������4���������ڲ�ͬ�����²�õĺϳɰ���Ӧ�����ʣ����з�Ӧ������ ��
A��v(H2)��0.1 mol��L��1��min��1 B��v(N2)��0.1 mol��L��1��min��1
C��v(NH3)��0.15 mol��L��1��min��1 D��v(N2)��0.002mol��L��1��min��1
��3����һ�����ȡ��ݻ�������ܱ������з������淴Ӧ��N2(g)��3H2(g) 2NH3(g) ��H��0�����и�����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬����
��
2NH3(g) ��H��0�����и�����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬����
��
A�������������ܶȱ��ֲ���
B���������¶Ȳ��ٱ仯
C������1mol N��N����ͬʱ������6 mol N��H��
D����Ӧ����N2��H2�����NH3������֮��1�U3�U2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com