
��14�֣�ijѧ����0.1000 mol��L��1��NaOH����Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷֽ�Ϊ���¼�����
A����ȡ20 mL����������Һע��ྻ����ƿ��������2��3�η�̪��
B���ñ���Һ��ϴ�ζ���2��3�Σ�
C����ʢ�б���Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ��
D��ȡ��NaOH��Һע���ʽ�ζ������̶�0����2��3 cm��
E������Һ����0��0���¿̶ȣ����¶�����
F������ƿ���ڵζ��ܵ����棬�ñ�NaOH��Һ�ζ����յ㲢���µζ���Һ��Ŀ̶ȡ�
�ʹ�ʵ�������գ�
��1����ȷ���������˳����(�������ĸ��д)______________________________��
��2������B���������Ŀ����_______________________________________��
��3��ʵ���������ֿ��ƻ������۾�ע�� ___________________��ֱ���ζ��յ㡣�жϵ����յ��������________________________________��
��4��ijѧ������3��ʵ��ֱ��¼�й��������±���
| �ζ� ���� | ������Һ�����(mL) | 0.100 0 mol��L��1NaOH�����(mL) | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���(mL) | ||
| ��һ�� | 20.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 20.00 | 1.56 | 30.30 | 28.74 |
| ������ | 20.00 | 0.22 | 26.31 | 26.09 |
��1��ABDCEF��BDCEAF ��2����ȥ���ڵζ��ܱ��ϵ�ˮ����ֹˮϡ�ͱ���Һ
��3����ƿ����Һ����ɫ�仯 ��Һ����ɫ����ɫ��dz���ұ��ְ�����ڲ���ɫ
��4��0.1305mol/L ��5�� 10 ��6��B��C��E
���������������1���ζ�����˳��Ϊ��ȡ����Һ�������װ���Һ֮��װ��Һ����ϴ��װҺ�������ݡ���Һ�桢���������ζ��������ظ�����������ȷ���������˳����ABDCEF��
��2���ñ�NaOH��Һ��ϴ�ζ���2��3�Σ���ȥ���ڵζ��ܱ��ϵ�ˮ����ֹˮϡ�ͱ���Һ��
��3��ʵ���������ֿ��ƻ������۾�ע����ƿ����Һ����ɫ�仯��ֱ���ζ��յ㡣�жϵ����յ����������Һ����ɫ����ɫ��dz���ұ��ְ�����ڲ���ɫ��
��4�������NaOH��Һ�����ƽ��ֵΪ26.10mL���������������ʵ���Ũ��Ϊ��26.10��0.100����20=0.1305mol/L��
��5�����û�ѧ��Ӧ�����кͷ�Ӧ���㣬c(OH-)=1��10-4 mol��L-1,pH=10��
��6��A. ��ƿ����Һ����ɫ�ո�����ɫ��Ϊdz��ɫ��ֹͣ�ζ������±���Һ������٣��ⶨ���ƫ�ͣ�B. ��ʽ�ζ���������ˮϴ��������ע���Һ�����±�ҺŨ�Ƚ��ͣ�����������ⶨ���ƫ�ߣ�C. �ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ������������ⶨ���ƫ�ߣ�D. �ζ�ǰ������ȷ���ζ����ӵζ��ܶ�������С�ˣ��ⶨ���ƫ�ͣ�E�� ʵ���У��ô�ʢװ����Һ��ϴ��ƿ�����±�Һ�������ⶨ���ƫ�ߣ�ѡB��C��E��
���㣺��������к͵ζ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���IJ�Ϊ�����ʱ���õ��Ӳ�������ɵĵ�����Ŀ��
| A��2�� | B��8�� | C��18�� | D��32�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������ʵ�����ֻ���¶��йص���
| A���������ܽ�� | B��ˮ�����ӻ� |
| C��������ص������� | D������ĵ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������ˮ��Һ�е��뷽��ʽ��д��ȷ����
A��HClO H++Cl -+ O 2- H++Cl -+ O 2- |
B��H2CO3 2H++ CO32- 2H++ CO32- |
| C��NaHSO4��Na++HSO4�C |
D��NH3��H2O NH4+��OH�� NH4+��OH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����˵����ȷ����
| A��ǿ�����һ��������ˮ |
| B�������ں�ˮ�з����绯��ʴʱ�����Ǹ��������� |
| C���Կ��淴Ӧ�������¶�һ������v(��)>v(��) |
| D����ͬŨ��ʱ��ǿ����ˮ�ĵ���̶ȱ������ˮ�ĵ���̶�С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��14�֣�ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
�ٽ���ʽ�ζ���������ˮϴ������鲻©ˮ�����ô�����Һ��ϴ����ע�������Һ�����ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0���̶����µ�λ�ã����¶���������ƿ������ˮϴ�����ô�����Һ��ϴ��ƿ2��3�Σ��Ӽ�ʽ�ζ����з���25��00 mL������Һ����ƿ�С�
�ڽ���ʽ�ζ���������ˮϴ������鲻©ˮ������������ע��0��1000 mol/L�����ᣬ���ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0���̶����µ�λ�ã����¶�����
������ƿ�е����̪��ָʾ�������еζ����ζ���ָʾ���պñ�ɫ������30 s����ɫ���ٱ仯�����������������ΪV1 mL��
���ظ����Ϲ��̣����ڵζ�����������ƿ�м���5 mL������ˮ�����������������ΪV2 mL��
�Իش��������⣺
��1����ƿ�е���Һ��_____ɫ��Ϊ_____ɫʱ��ֹͣ�ζ���
��2���ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�_____��
A���ζ�����Һ��ı仯 B����ƿ����Һ��ɫ�ı仯
��3����С���ڲ�����еĴ�����__________���ɴ���ɵIJⶨ���_____(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)
��4�������ȱ�ٵIJ�����__________��
��5����ͼ����ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ_____ mL��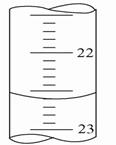
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
11��)��1����ͼ�ֱ����¶ȼơ���Ͳ���ζ��ܵ�һ���֣�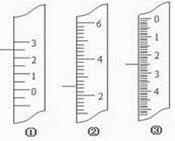
����ͲӦ���� ����Ϊ mL���ζ���Ӧ���� ����Ϊ mL���¶ȼ�Ӧ���� ��
��2��ʵ������һƿ�����Ȼ��Ƶ��������ƹ����Լ���Ϊ����֤�䴿�ȣ�����֪Ũ�ȵ�������еζ����Իش��������⣺
�ٳ�ȡһ�����������Լ�����100mL��Һ��
�ڽ�������װ��25��00mL �ζ����У�����Һ��λ���� �������¿̶ȡ�
��ȡ20��00mL����Һ�����ⶨ������ʵ���������Ҫ������ ��
�� �Լ���ָʾ����
������ʵ������������������ƺ���ƫ�ߵ��� ��
| A���ζ�ǰ�ζ��ܼ��������ݣ��ζ���ζ��ܼ��������� |
| B���к͵ζ�ʱ����ƿ������������ˮ |
| C����ʽ�ζ���������ˮϴ���ñ�Һ��ϴ2-3�� |
| D����ȡ�ζ����յ����ʱ�����ӿ̶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������������ϩ��������Ļ�ѧ�Լ���
| A��NaOH ��Һ | B��HCl | C���Ҵ� | D�����Ը��������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������ʱ��뱣������ɫ�Լ�ƿ�е���
����ˮ �ڰ�ˮ ��Ũ���� ���������� ����ˮ
| A��ȫ�� | B���٢ۢ� | C���٢ڢ� | D���٢ڢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com