��2011?��������ģ��H
2O
2����Ҫ�Ļ�ѧ�Լ�����ʵ���Һ�����ʵ����Ӧ�ù㷺��
��Ӧ���䲻�ȶ��ԣ�H
2O
2�ɱ������о������Է�Ӧ���ʵ�Ӱ�죮
��1���H
2O
2��MnO
2���·ֽ�Ļ�ѧ����ʽ
��
��2��H
2O
2��������Ҫ�ϸ�������Fe
2+��Fe
3+������ᷢ������������Ӧ���ӿ���ֽ⣬��Fe
2+��Fe
3+���Ե������ֲ��䣮
��2Fe
2++H
2O
2+2H
+�T2Fe
3++2H
2O
��
2Fe3++H2O2=2Fe2++O2��+2H+
2Fe3++H2O2=2Fe2++O2��+2H+
�������ӷ���ʽ��
��3���ӣ�1���ͣ�2������������������Ԫ����Ѱ��H
2O
2�ֽ�Ĵ��������о���ֵ
b
b
��
a������Ԫ��b������Ԫ��c������Ԫ��
��H
2O
2��ˮ��Һ�������ԣ�д��һ����������ȡH
2O
2�Ļ�ѧ����ʽ
Na2O2+2HCl=2NaCl+H2O2
Na2O2+2HCl=2NaCl+H2O2
��
��H
2O
2��ʹ����ͨ�����������Ⱦ��
��1����ҵ������Cl
2+H
2O
2�T2HCl+O
2���ȣ��ڴ˷�Ӧ�б�������������
H2O2
H2O2
��
��2������ij��Һ���軯���CN
-����������з�Ӧ��CN
-+H
2O
2=HCO3-
=HCO3-
+NH
3��
����ʵ���ҳ�������KMnO
4��Һ�ⶨH
2O
2��Ũ�ȣ�
ȡ10.00mLH
2O
2��Һ��Ʒ���ܶȽ���Ϊ1g/mL������ƿ�У���ˮ��50.00mL��������0.10mol/L KMnO
4��Һ40.00mL����ζ��յ㣮��ԭ��Ʒ��H
2O
2������������
3.4%��
3.4%��
��
����֪��2KMnO
4+5H
2O
2+3H
2SO
4�TK
2SO
4+2MnSO
4+5O
2��+8H
2O����







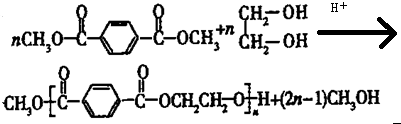
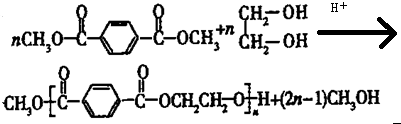
 �ϳ�
�ϳ� ������ͼ����ע����Ӧ������
������ͼ����ע����Ӧ������