
(2)���ڴ��������ʹ�õ������ݱ���ȷ����ƫ�����___________��
A.����Ͳ��ȡһ����Һ��ʱ������Һ�����
B.ʹ������ƿ������Һʱ������Һ����ж��ݣ�������Һ��Ũ��
C.�к͵ζ��ﵽ�յ�ʱ�����ӵζ���Һ��Ķ���
(3)������ʵ�飺����������ȡ ����ϩ����ȡ ��ʯ�͵ķ��� ��������������ȡ�����в���Ҫ�¶ȼƵ���___________(��д���)��
(4)����ʵ����ѡ�õ�����������Լ����ۺ�������___________��
A.�ü�ʽ�ζ�����ȡ25.00mL����KMnO4��Һ
B.�ⶨ��Һ��pHʱ���ò���պȡ��Һ������������ˮʪ�����pH��ֽ�ϣ��������ɫ�����б�ɫ
C.pH��ȵ�NaOH�Ͱ�ˮ��ֻ��pH��ֽ������ˮ�Ϳ��Լ���
(1)7.2 (2)C (3)�٢� (4)C
���������⿼�黯ѧʵ��Ļ���������һЩ����������ʹ�÷�����Ҫ������һ����ʵ�������۲�������
(1)�罫��Ʒ�����̣������̷���8 g���룬����Ϊ0.8g������ƽ���ϵm����m��+m��
�� 8��m��+0.8 ����m����7.2 g
(2)Ҫע����Ͳ�����̶����ϣ��ζ�������̶����ϣ�����ƿֻ��һ���̶���
(3)ʵ��������ϩ��Ҫ�¶ȼƿ�����170�棻ʯ�͵ķ���Ҫ���¶ȼ�����ͬ�е����֣�������ʵ�鲻���¶ȼ�
(4)��ʽ�ζ�����һ����Ƥ���ױ�KMnO4��������KMnO4ֻ������ʽ�ζ�����ȡ��pH��ֽ�Dz�����ʪ�ģ�pH��ȵ�NaOH�Ͱ�ˮϡ�͵����ʱ��pH�仯����ȣ��ʿ�����pH��ֽ������ߡ�


 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2�����ڴ��������ʹ�õ������ݱ���ȷ����ƫ�����________g��?
A.����Ͳ��ȡһ����Һ��ʱ������Һ�����?
B.ʹ������ƿ������Һʱ������Һ����ж��ݣ�������Һ��Ũ��?
C.�к͵ζ��ﵽ�յ�ʱ�����ӵζ���Һ��Ķ���?
��3��������ʵ�飺����������ȡ������ϩ����ȡ����ʯ�͵ķ���������������ȡ�����в���Ҫ�¶ȼƵ���______________����д��ţ���
��4������ʵ����ѡ�õ�����������Լ����ۺ�������________________��
A.�ü�ʽ�ζ�����ȡ25.00 mL����KMnO4��Һ
B.�ⶨ��Һ��pHʱ���ýྻ������IJ�����պȡ��Һ������������ˮ��ʪ����pH��ֽ�ϣ��������ɫ�����б�ɫ?
C. pH��ȵ�NaOH�Ͱ�ˮ��ֻ��pH��ֽ������ˮ�Ϳ��Լ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(11��)��ѧ��ȤС���������ʵ�鷽�����ⶨ�Ѳ��ֱ��ʵ�ijС�մ���Ʒ��Na2CO3������������
[����һ]��ȡһ��������Ʒ�����������м��������غ���ȴ������ʣ��������������㡣
(1)�����з�����Ӧ�Ļ�ѧ����ʽΪ��
________________________________________________________________________��
(2)ʵ��������������ص�Ŀ���ǣ�
________________________________________________________________________��
(3)ʵ����ȷ�����������صķ����ǣ�
________________________________________________________________________��
(4)�����ȹ������������о���Ž����������õĽ��________(�ƫ����ƫС�����䡱)��
[������]��ȡһ��������Ʒ������С�ձ��У�������ˮ�ܽ⣻��С�ձ��м�������Ba(OH)2��Һ�����ˣ�ϴ�ӣ���������������������������㡣(��֪��Ba2����OH����HCO3��===BaCO3����H2O)
(1)���˲����У������ձ���©���⣬��Ҫ�õ��IJ�������Ϊ______________��
(2)ʵ�����жϳ����Ƿ���ȫ�ķ�����
________________________________________________________________________________________________________________________________________��
(3)ʵ����ϴ�ӳ����IJ�����
________________________________________________________________________��
(4)ʵ�����жϳ����Ƿ�ϴ�Ӹɾ��ķ�����
_______________________________________________________________________________________________________________________________________________��
[������]
����ͼ��ʾװ�ý���ʵ�飺
(1)Bװ������ʢ�Լ���________��Dװ�õ�������______________����Һ©����________(��ܡ����ܡ�)���������ϡ�������ʵ�顣
(2)ʵ��ǰ��ȡ17.9 g��Ʒ��ʵ�����Cװ������8.8 g������Ʒ��Na2CO3����������Ϊ________��
(3)���ݴ�ʵ���õ����ݣ��ⶨ���������Ϊʵ��װ�û�����һ������ȱ���ǣ�____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��߿���ѧһ�ָ�ϰ����ѧʵ�������ר���ۺϲ��ԣ��ս̰棩 ���ͣ�ʵ����
(11��)��ѧ��ȤС���������ʵ�鷽�����ⶨ�Ѳ��ֱ��ʵ�ijС�մ���Ʒ��Na2CO3������������
[����һ]��ȡһ��������Ʒ�����������м��������غ���ȴ������ʣ��������������㡣
(1)�����з�����Ӧ�Ļ�ѧ����ʽΪ��
________________________________________________________________________��
(2)ʵ��������������ص�Ŀ���ǣ�
________________________________________________________________________��
(3)ʵ����ȷ�����������صķ����ǣ�
________________________________________________________________________��
(4)�����ȹ������������о���Ž����������õĽ��________(�ƫ����ƫС�����䡱)��
[������]��ȡһ��������Ʒ������С�ձ��У�������ˮ�ܽ⣻��С�ձ��м�������Ba(OH)2��Һ�����ˣ�ϴ�ӣ���������������������������㡣(��֪��Ba2����OH����HCO3��===BaCO3����H2O)
(1)���˲����У������ձ���©���⣬��Ҫ�õ��IJ�������Ϊ______________��
(2)ʵ�����жϳ����Ƿ���ȫ�ķ�����
________________________________________________________________________________________________________________________________________��
(3)ʵ����ϴ�ӳ����IJ�����
________________________________________________________________________��
(4)ʵ�����жϳ����Ƿ�ϴ�Ӹɾ��ķ�����
_______________________________________________________________________________________________________________________________________________��
[������]
����ͼ��ʾװ�ý���ʵ�飺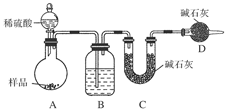
(1)Bװ������ʢ�Լ���________��Dװ�õ�������______________����Һ©����________(��ܡ����ܡ�)���������ϡ�������ʵ�顣
(2)ʵ��ǰ��ȡ17.9 g��Ʒ��ʵ�����Cװ������8.8 g������Ʒ��Na2CO3����������Ϊ________��
(3)���ݴ�ʵ���õ����ݣ��ⶨ���������Ϊʵ��װ�û�����һ������ȱ���ǣ�____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��߿���ѧһ�ָ�ϰ����ѧʵ�������ר���ۺϲ��ԣ��ս̰棩 ���ͣ�ʵ����
(11��)��ѧ��ȤС���������ʵ�鷽�����ⶨ�Ѳ��ֱ��ʵ�ijС�մ���Ʒ��Na2CO3������������
[����һ]��ȡһ��������Ʒ�����������м��������غ���ȴ������ʣ��������������㡣
(1)�����з�����Ӧ�Ļ�ѧ����ʽΪ��
________________________________________________________________________��
(2)ʵ��������������ص�Ŀ���ǣ�
________________________________________________________________________��
(3)ʵ����ȷ�����������صķ����ǣ�
________________________________________________________________________��
(4)�����ȹ������������о���Ž����������õĽ��________(�ƫ����ƫС�����䡱)��
[������]��ȡһ��������Ʒ������С�ձ��У�������ˮ�ܽ⣻��С�ձ��м�������Ba(OH)2��Һ�����ˣ�ϴ�ӣ���������������������������㡣(��֪��Ba2����OH����HCO3��===BaCO3����H2O)
(1)���˲����У������ձ���©���⣬��Ҫ�õ��IJ�������Ϊ______________��
(2)ʵ�����жϳ����Ƿ���ȫ�ķ�����
________________________________________________________________________________________________________________________________________��
(3)ʵ����ϴ�ӳ����IJ�����
________________________________________________________________________��
(4)ʵ�����жϳ����Ƿ�ϴ�Ӹɾ��ķ�����
_______________________________________________________________________________________________________________________________________________��
[������]
����ͼ��ʾװ�ý���ʵ�飺
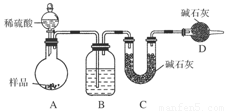
(1)Bװ������ʢ�Լ���________��Dװ�õ�������______________����Һ©����________(��ܡ����ܡ�)���������ϡ�������ʵ�顣
(2)ʵ��ǰ��ȡ17.9 g��Ʒ��ʵ�����Cװ������8.8 g������Ʒ��Na2CO3����������Ϊ________��
(3)���ݴ�ʵ���õ����ݣ��ⶨ���������Ϊʵ��װ�û�����һ������ȱ���ǣ�____________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com