
����Ŀ����ͼΪ��25mL 0.1mol��L1NaOH��Һ����εμ�0.2mol��L1CH3COOH��Һ��������ҺpH�ı仯���ߡ���ش�
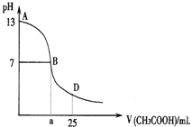
(1)B����Һ������,���˾ݴ���Ϊ,��B��ʱNaOH��CH3COOHǡ����ȫ��Ӧ,���ֿ����Ƿ���ȷ?(ѡ��ǡ���)_________.������ȷ,�����ǡ����ȫ��Ӧ�ĵ�����AB���仹��BD������?__________________(����ȷ,���ʲ���)
(2)AB����,c(OH)>c(H+),��c(OH)��c(CH3COO)��С��ϵ��_______________
A.c(OH)һ������c(CH3COO) B.c(OH)һ��С��c(CH3COO)
C.c(OH)һ������c(CH3COO) D.c(OH)���ڡ�С�ڻ����c(CH3COO)
(3)��D��ʱ,��Һ��c(CH3COO)+c(CH3COOH)_________2c(Na+)(��>������<����=��)
(4)������,��VmL��0.1000mol��L1����������Һ��μ��뵽20.00mL��0.1000mol��L1������Һ��,��ַ�Ӧ���ش���������.(������Һ����ı仯)
�������ҺpH=7,��ʱV��ȡֵ____________20.00(�>������<����=��),����Һ��c(Na+)��c(CH3COO)��c(H+)��c(OH)�Ĵ�С��ϵΪ________________________.
�����V=40.00,���ʱ��Һ��c(OH)c(H+)c(CH3COOH)=__________________mol��L1
���𰸡��� AB D = < c(Na+)=c(CH3COO)>c(H+)=c(OH) 1/30��0.033mol/L
��������
��1��NaOH��CH3COOHǡ����ȫ��Ӧ�����ɴ����ƣ�������Ϊǿ�������Σ�ˮ�����Һ�Լ��ԣ�pH��7��
��2����AB�����ڣ�����CH3COOH��NaOHǡ����ȫ��Ӧ�Լ�CH3COOH���㡢����������Һ�������ֿ����ԣ�
��3����D��ʱ��NaOH��CH3COOH��Ӧ��ʣ��CH3COOH����Һ�����Ϊ��Ũ�ȵ�CH3COOH��CH3COONa�Ļ���
��4���������NaOH��Һ�����20.00mL������ǡ����ȫ��Ӧ���ɴ����ƣ���Һ�ʼ��ԣ���Ϊ���ԣ�����������������Һ���С��20.00mL��
��1��NaOH��CH3COOHǡ����ȫ��Ӧ��NaOH+CH3COOH=CH3COONa+H20�����ɵĴ�����Ϊǿ�������Σ���Һ�Լ��ԣ�pH>7������AB֮�䣬
�ʴ�Ϊ��AB��
��2����AB�����ڣ�c��OH-��>c��H-����˵����Һ�Լ��ԣ���NaOH��CH3COOHǡ�÷�Ӧʱ���Լ��ԣ���ʱ��Һ�е�����Ϊ�����ƣ�������ˮ��̶Ƚ�С����Һ�������ԣ���c��OH-��С��c��CH3COO-������NaOH��CH3COOH��Ӧ��ʣ��NaOH����Һ��Ȼ�Լ��ԣ���ʱ��ʣ���NaOH���ܴ���c��OH-������c��CH3COO-������ȻҲ�п�����c��OH-��=c��CH3COO-����
�ʴ�Ϊ��D��
��3����D��ʱ����Ӧ��CH3COOHʣ�࣬��Һ�ɵ�Ũ�ȵ�CH3COOH��
CH3COONa��ɣ����������غ㣬��ʱ��c��CH3COO-��+c��CH3COOH��=2c��Na+����
�ʴ�Ϊ=��
��4����CH3COOH��������ʣ�����̶Ȳ���NaOH��ǿ����ʣ���ȫ���룬��Ӧ���ɵ���������ǿ�������Σ�ˮ��ʼ��ԣ�����Һ������pH=7�����ټӼ���Գ����£���V mL��0.1000molL-1����������Һ��μ��뵽20.00mL��0.1000molL-1������Һ�У���ַ�Ӧ��V<20.00mL��Һ������pH=7��c��H+��=c��OH-�������ݵ���غ�c��Na+��+c��H+��=c��CH3COO-��+c��OH-����c��H+��=c��OH-������Һ�е�����Ϊ��������Һ��ˮ�ĵ��������ģ�����c��Na+��=c��CH3COO-��>c��H+��=c��OH-����
�ʴ�Ϊ<��c��Na+��=c��CH3COO-��>c��H+��=c��OH-����
�ڸ��ݵ���غ�c��Na+��+c��H+��=c��CH3COO-��+c��OH-���������غ�c��Na+��=2[c��CH3COO-��+c��CH3COOH��]���õ�c��H+��+c��CH3COO-��+2c��CH3COOH��=c��OH-������c��OH-��-c��H+��-c��CH3COOH��=c��CH3COO-��+c��CH3COOH������Ӧ����Һ�������Ϊ60mL����c��CH3COO-��+c��CH3COOH���T0.1000mol/L��20mL��60mL=1/30mol/L��0.033mol/L��
�ʴ�Ϊ1/30��0.033��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��ҡ��������ֱ���![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() �����е�������ɵĻ�������Է�������ͼת����
�����е�������ɵĻ�������Է�������ͼת����
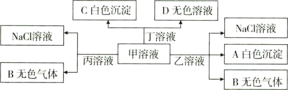
����˵���������![]()
![]()
A.��Ϊ![]()
B.�ܴ�����������Һ���е����ӣ�![]() ��
��![]() ��
��![]()
C.����Һ�����ӵĵ�����ϵ��![]()
D.����Һ�еμ���������Һ��Ӧ�����ӷ���ʽ��![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��ijͬѧ�����һ��ȼ�ϵ�ز�̽���ȼҵԭ�������������������װ����XΪ�����ӽ���Ĥ���밴Ҫ��ش����������
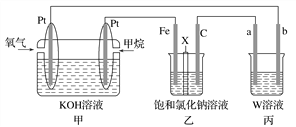
(1)ʯī�缫(C)��________�������м���ȼ�ϵ�صĸ�����ӦʽΪ______________________________��
(2)������2.24 L(���)����������װ�������缫�����ɵ��������(���)Ϊ________ L��
(3)������CuSO4��ҺΪ�������Һ���д�ͭ(��Al��Zn��Ag��Pt��An������)�ĵ�⾫��������˵����ȷ����________(����ĸ)��
A��a�缫Ϊ��ͭ
B����ͭ�ӵ�Դ������������ԭ��Ӧ
C��CuSO4��Һ��Ũ�ȱ��ֲ���
D������������ɻ���Ag��Pt��Au�Ƚ���
(4)��������ϡH2SO4Ϊ�������Һ���缫����bΪ��������ʹ����������һ�����ܵ�����Ĥ���õ缫��ӦʽΪ__________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ҫ����д���пհף�
(1)�����¾�Ϊ0.02mol/L������������ƣ��������Ϻ���Һ��pH=________
(2)�����£�pH��Ϊ11��Na2CO3��NaOH��Һ�У���ˮ�������c(OH-)֮��Ϊ_____________
(3)��t ��ʱ��AgBr��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪AgCl��Ksp=4��10-10���� AgCl(s)+Br-(aq)![]() AgBr(s)+Cl-(aq)��ƽ�ⳣ��K=_______________��
AgBr(s)+Cl-(aq)��ƽ�ⳣ��K=_______________��

(4)��֪��Ԫ��H2A��ˮ�д������µ��룺H2A=H++HA-��HA-![]() H++A2-���ش��������⣺
H++A2-���ش��������⣺
��NaHA��Һ��________(������������������������������ȷ����)�ԣ�������____________��
��ij�¶��£���10 mL��0.1 mol/L NaHA��Һ�м���0.1 mol/L KOH��ҺV mL�����ԣ���ʱ��Һ�����¹�ϵһ����ȷ����________(��д��ĸ)��
a.��ҺpH=7 b.ˮ�����ӻ�Kw=c2(OH-) c.V=10 d.c(K+)<c(Na+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����С����ʵ�����Ʊ��������������йذ���������̽����
![]() ��С��ͬѧ������ʯ�����Ȼ�淋Ļ������ȡ����İ�����
��С��ͬѧ������ʯ�����Ȼ�淋Ļ������ȡ����İ�����
![]() Ӧ��ѡ�õ�������װ����
Ӧ��ѡ�õ�������װ����![]() ����ĸ
����ĸ![]() ______ ��
______ ��
![]() ��ʯ�����Ȼ�立�Ӧ���ɰ����Ļ�ѧ����ʽΪ ______ ��
��ʯ�����Ȼ�立�Ӧ���ɰ����Ļ�ѧ����ʽΪ ______ ��
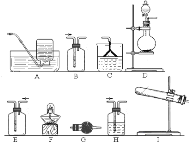
![]() ��С��ͬѧ�����ͼ��ʾװ��̽�������Ļ�ԭ�ԣ�
��С��ͬѧ�����ͼ��ʾװ��̽�������Ļ�ԭ�ԣ�

���������̻�ͬʱ���������Ӧ��д�����������û���Ӧ�Ļ�ѧ����ʽ ______ ![]() ��ʵ��ʱͨ�백�������ʹ��죬����ƿ�л���ְ��̣������ʵĻ�ѧʽΪ ______ ��
��ʵ��ʱͨ�백�������ʹ��죬����ƿ�л���ְ��̣������ʵĻ�ѧʽΪ ______ ��
![]() ��С��ͬѧ�����Ͷ�����̼Ϊԭ���Ʊ�̼����泥�
��С��ͬѧ�����Ͷ�����̼Ϊԭ���Ʊ�̼����泥�
![]() ��ͬѧ�Ƚ�������̼ͨ��ˮ�У�����ܽ����ͨ�백������ͬѧ�Ƚ�����ͨ��ˮ�У�����ܽ����ͨ�������̼�����ʵķ����� ______
��ͬѧ�Ƚ�������̼ͨ��ˮ�У�����ܽ����ͨ�백������ͬѧ�Ƚ�����ͨ��ˮ�У�����ܽ����ͨ�������̼�����ʵķ����� ______ ![]() ����������������
����������������![]() ��ԭ���� ______ ��
��ԭ���� ______ ��
![]() �����������
�����������![]() �ķ���Ϊ ______ ��
�ķ���Ϊ ______ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Al��Cl2��Al2O3��HCl(aq)��Al(OH)3��NaOH(aq)�������ʣ�����֮������ͼ��ʾ��ת����ϵ��ͼ��ÿ�������˵�����֮�䶼���Է�����Ӧ�������ƶϲ��������ǣ� ��
![]()
A.X����ΪAl��Cl2
B.Yһ��ΪNaOH(aq)
C.Nһ����HCl(aq)
D.Q��Z�е�һ�ֱ�ΪAl2O3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и������ӣ���������������һ���ܹ������������
A.��pH=0����Һ�У�Fe2+��Mg2+��SO![]() ��Cl-
��Cl-
B.��ˮ�������c(H+)=1��10-12mol/L����Һ�У�NH![]() ��K+��Na+��NO
��K+��Na+��NO![]()
C.��A1C13��Һ�У�SO![]() ��Na+��CO
��Na+��CO![]() ��AlO
��AlO![]()
D.�ڵμ�ʯ����Һ��ʺ�ɫ����Һ�У�I-��Na+��S2-��AlO![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ֲ�ﻨ֭����ȡ��һ���л���HIn�������ᡢ��ָʾ������ˮ��Һ�д��ڵ���ƽ�⣺HIn����ɫ��![]() H+ + In-����ɫ����H��0������ƽ����Ͳ���ȷ����
H+ + In-����ɫ����H��0������ƽ����Ͳ���ȷ����
A.�����¶�ƽ�����������ƶ�
B.���������ƽ�����淽���ƶ�����Һ�Ժ�ɫ
C.����NaOH��Һ��ƽ�����������ƶ�����Һ�Ի�ɫ
D.����NaHSO4��Һ��ƽ�����������ƶ�����Һ�Ի�ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����¶Ȳ���������£��ں��ݵ������н������з�Ӧ��N2O4(g)![]() 2NO2(g)����N2O4��Ũ����0.1mol��L-1����0.07mol��L-1��Ҫ15s����ôN2O4��Ũ����0.07mol��L-1����0.05mol��L-1����ķ�Ӧʱ�䣨 ��
2NO2(g)����N2O4��Ũ����0.1mol��L-1����0.07mol��L-1��Ҫ15s����ôN2O4��Ũ����0.07mol��L-1����0.05mol��L-1����ķ�Ӧʱ�䣨 ��
A.����5sB.����10sC.��10sD.����10s
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com