
| ʵ����� | ʵ������ | ʵ���� |
| A | ��AgNO3��Һ | �а�ɫ�������� |
| B | ������NaOH��Һ������ | �ռ�������1.12L��������ɱ�״���µ������ |
| C | ������BaCl2��Һ�������ó�������ϴ�ӡ������������������м�����ϡ���ᣬȻ�������� | ��һ�γ�����������Ϊ6.27g���ڶ��γ�����������Ϊ2.33g |
���� A����AgNO3��Һ�а�ɫ�������ɣ�˵����Һ�п�����Cl-��SO42-��CO32-��
B��������NaOH��Һ�����ȣ��ռ�������1.12L��˵����Һ��һ����NH4+�����ɼ����笠����ӵ����ʵ���Ϊ0.05mol��
C��������BaC12��Һʱ����Һ�г�����������������п��������ᱵ��̼�ᱵ�������߶��У����Ƴ�ԭ��Һһ��û��Ba2+��
�����ó�������ϴ�ӡ������������������м�����ϡ���ᣬȻ����������һ�γ�������Ϊ6.27g���ڶ��γ�������Ϊ2.33g�����������������ܽ⣬˵���ó���һ�������ᱵ��̼�ᱵ�Ļ���˵���������һ����̼������������̼�����þ���Ӳ��ܹ��棬˵���������һ��û��þ���ӣ�2.33gΪ���ᱵ���������������ӵ����ʵ���Ϊ0.01mol��3.94gΪ̼�ᱵ�������̼������ӵ����ʵ���Ϊ0.02mol�������������ӵ���غ㣬笠����ӵ����ʵ���Ϊ0.05mol����������ӵ����ʵ���Ϊ0.01mol��̼������ӵ����ʵ���Ϊ0.02mol����֪�û����һ������K+��Cl-�Ƿ���ڲ����ж���
��� �⣺A����AgNO3��Һ�а�ɫ�������ɣ�˵����Һ�п�����Cl-��SO42-��CO32-��
B��������NaOH��Һ�����ȣ��ռ�������1.12L��˵����Һ��һ����NH4+�����ɼ����笠����ӵ����ʵ���Ϊ0.05mol��
C��������BaC12��Һʱ����Һ�г�����������������п��������ᱵ��̼�ᱵ�������߶��У����Ƴ�ԭ��Һһ��û��Ba2+��
�����ó�������ϴ�ӡ������������������м�����ϡ���ᣬȻ����������һ�γ�������Ϊ6.27g���ڶ��γ�������Ϊ2.33g�����������������ܽ⣬˵���ó���һ�������ᱵ��̼�ᱵ�Ļ���˵���������һ����̼������������̼�����þ���Ӳ��ܹ��棬˵���������һ��û��þ���ӣ�2.33gΪ���ᱵ���������������ӵ����ʵ���Ϊ0.01mol��3.94gΪ̼�ᱵ�������̼������ӵ����ʵ���Ϊ0.02mol�������������ӵ���غ㣬笠����ӵ����ʵ���Ϊ0.05mol����������ӵ����ʵ���Ϊ0.01mol��̼������ӵ����ʵ���Ϊ0.02mol����֪�û����һ������K+��Cl-�Ƿ���ڲ����ж���
��1������ʵ��A�ж�Cl-��һ�����ڣ��ʴ�Ϊ������ȷ����
��2���������һ�������ڵ������ǣ�Ba2+��Mg2+���ʴ�Ϊ��Ba2+��Mg2+��
��3��ʵ��B�з�����Ӧ�����ӷ���ʽΪNH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O���ʴ�Ϊ��NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O��
��4��ͨ��ʵ���֪��Һ�п϶����ڵ�������NH4+��CO32-��SO42-�������㣬NH4+�����ʵ���Ũ��Ϊ0.5 mol/L��CO32-��SO4+�����ʵ���Ũ�ȷֱ�Ϊ0.2 mol/L��0.1 mol/L�����ݵ���غ�ã�K+һ�����ڣ�
�𣺴��ڣ�ͨ��ʵ���֪��Һ�п϶����ڵ�������NH4+��CO32-��SO42-�������㣬NH4+�����ʵ���Ũ��Ϊ0.5 mol/L��CO32-��SO4+�����ʵ���Ũ�ȷֱ�Ϊ0.2 mol/L��0.1 mol/L�����ݵ���غ��K+һ�����ڣ�
���� ���⿼���˳����ļ������ӵļ��鷽������Ŀ�Ѷ��еȣ�����ʱ�ر�ע��K+���ж�Ӧ�ø��ݵ���غ��жϣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ữ��BaCl2��Һ���ж��������ƹ����Ƿ������� | |
| B�� | ������������Һ���Ƿ������ɫ�������ж���Һ���Ƿ��������� | |
| C�� | �ɼ���������ͭ��Ȼ���������ȥ�Ȼ�������Һ�е������Ȼ��� | |
| D�� | �������ʯ��ˮ���Ƿ��а�ɫ����������̼������Һ��̼��������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���»������ձ���ڵ�һ�ַ��Ӽ������������ڵ������� | |
| B�� | ���»����Ƚ����������»���Խǿ�����ʵ��۵�ͷе�Խ�� | |
| C�� | �������һ�ֽ�ǿ�ķ��Ӽ���������ֻ�ܴ����ڷ���֮�� | |
| D�� | �γ����ʱ���뺬����ԭ�ӣ�������ԭ�����ߵ�ԭ�ӱ�����к�ǿ�ĵ縺�ԡ���С��ԭ�Ӱ뾶 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��� | ��Ӧ�� | ���� |
| �� | KMnO4��H2O2��H2SO4 | K2SO4��MnSO4�� |
| �� | Cl2��FeBr2 | FeCl3��Br2 |
| �� | MnO2�� | Cl2��Mn2+�� |
| A�� | �ڢ��鷴Ӧ���������ΪH2O | |
| B�� | �ڢ��鷴Ӧ������1mol Cl2��ת�Ƶ���4mol | |
| C�� | ��������ǿ����˳��ΪMnO4-��Cl2��Fe3+��Br2 | |
| D�� | �ڢ��鷴Ӧ��Cl2�� FeBr2�����ʵ���֮��Ϊ3��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
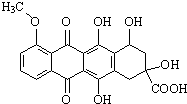
| A�� | ���ڷ����� | |
| B�� | �ܷ�����ȥ��Ӧ��������Ӧ | |
| C�� | ����������̼ԭ�Ӳ�������ͬһƽ���� | |
| D�� | 1mol���л���������NaOH��Һ��Ӧ������5molNaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢۢ� | B�� | �ڢۢ� | C�� | �ڢ� | D�� | ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 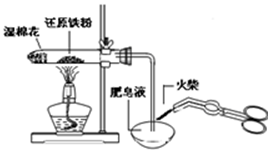 ����������ˮ������Ӧ���������� ����������ˮ������Ӧ���������� | |
| B�� |  �����Ʊ����ռ�һ������������İ��� �����Ʊ����ռ�һ������������İ��� | |
| C�� |  ���뻥�ܵ��е������Һ������ | |
| D�� | 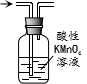 ��ȥ��������������ϩ�ô������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HNO3��NaCl��K2SO4 | B�� | KCl��NaOH��CuSO4 | ||
| C�� | BaCl2��NaOH��H2SO4 | D�� | FeCl3��Na2SO4��KCl |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com