
�����£���
0.1 mol/L��HA��Һ��0.1 mol/L��NaOH��Һ�������Ϻ�(���Ի�Ϻ���Һ����ı仯)����û����Һ��pH����8���Իش��������⣺(1)�����Һ��pH����8��ԭ����(�����ӷ���ʽ��ʾ)________��
(2)�����Һ����ˮ�������c(H+)________0.1 mol/L��NaOH��Һ����ˮ�������c(H+)(����ڡ�����С�ڡ����ߡ����ڡ�)��
(3)��������Һ��������ʽ�ľ�ȷ������(���������)��
c(Na+)��c(A��)��________mol/L��
c(OH��)��c(HA)��________mol/L��
 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д� һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ������2012�������ǰ��Ϣ����ѧ���� ���ͣ�021
|
�����йص������Һ���������ʵ���Ũ�ȹ�ϵ��ȷ���� | |
A�� |
�����£���0.1 mol��L��1��NH4Cl��Һ��0.05 mol��L��1��NaOH��Һ�������ϣ�c(Cl��)��c(Na+)��c(NH4+)��c(OH��)��c(H+) |
B�� |
�����£���CH3COOH��Һ�м���������NaOH���õ�pH��4�Ļ����Һ��c(Na+)��c(CH3COO��)��c(H+)��c(OH��) |
C�� |
Ũ�Ⱦ�Ϊ0.1 mol��L��1��Na2CO3��Һ��NaHCO3��Һ�������ϣ�c(Na+)��c(H+)��2c(CO32��)��c(OH��)��c(HCO3��) |
D�� |
�����£�pH��3��һԪ��HX��Һ��pH��11��һԪ��MOH��Һ�������ϣ�c(M+)��c(X��)��c(H+)��c(OH��) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ٻ����Һ����ˮ�������c(H+)__________0.2 mol��L-1 HCl��Һ����ˮ�������c(H+);(�������������=��)
����������Һ��������ʽ�ľ�ȷ����������������֣���
c(Cl-)-c(M+)= __________mol��L-1;
c(H+)-c(MOH)= __________ mol��L-1��
��2������������0.2 mol��L-1 MOH��Һ��0.1 mol��L-1 HCl��Һ�������ϣ���û����Һ��pH��7����˵������ͬ������MOH�ĵ���̶�__________MCl��ˮ��̶ȡ����������������=����
��3������������pH=3��HR��Һ��pH=11��NaOH��Һ�������ϣ���û����Һ��pH��7,������Һ��pH__________�������7������7������ȷ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ�����и߶��Ͻ�ѧ������⻯ѧ�Ծ��������棩 ���ͣ������
������������ճ������м�Ϊ�������ᣬ��һ��������CH3COOH��Һ�д��ڵ���ƽ�⣺CH3COOH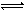 CH3COO��+H+ ��H��0
CH3COO��+H+ ��H��0
��1�������£�pH��5������Һ�У�c(CH3COO��)��______mol/L(��ȷֵ��Ҫ����ʽ���ػ���)��
��2�����з����п���ʹ0.10 mol��L-1 CH3COOH�ĵ���̶��������
a����������0.10 mol��L��1ϡ���� b������CH3COOH��Һ c����ˮϡ����0.010 mol��L��1
d���������������� e�����������Ȼ��ƹ��� f����������0.10 mol��L��1 NaOH��Һ
��3������������пͶ��������pH������3�Ĵ����������Һ�У�������ַ�Ӧ����ֻ��һ����Һ����п��ʣ�࣬�����������������V(����)_________V(����)������ڡ�����С�ڡ����ڡ���
��4����NaOH��Һ�ֱ�ζ�20.00mL0.1mol/L�����20.00mL0.1mol/L������Һ���õ���ͼ��ʾ�����ζ����ߣ���NaOH��Һ�ζ�������Һ�������� ���ͼ1����ͼ2����
��5�������£���0.1 mol/L�����0.1 mol/L��������Һ��ϣ�������ҺΪ���ԣ�������Һ�и����ӵ�Ũ�Ȱ��ɴ�С����Ϊ_______________________________��
��6����ͼ��ʾ��Һ��c(H��)��c(OH��)�Ĺ�ϵ
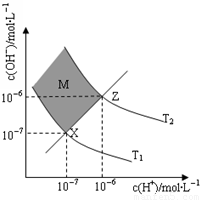
��M�����ڣ���Ӱ���֣������c(H��)______c(OH��)������ڡ�����С�ڡ����ڡ���
����T2�¶��£���pH��9 NaOH��Һ��pH��4 HCl��Һ��ϣ������û����Һ��pH��7����NaOH��Һ��HCl��Һ�������Ϊ ������Ϻ���Һ����ı仯���Բ��ƣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��8�֣������½�0.2 mol/L HCl��Һ��0.2 mol/L MOH��Һ��������(���Ի�Ϻ���Һ����ı仯)����û����Һ��pH��6���Իش��������⣺
(1)�ٻ����Һ����ˮ�������c(H+)________HCl��Һ����ˮ�������c(H+)��(�>������<������)
����������Һ��������ʽ�ľ�ȷ������(���������)��
c(Cl��)��c(M+)��__________mol/L��c(H+)��c(MOH)��________mol/L��
(2)����������0.2 mol/L MOH��Һ��0.1 mol/L HCl��Һ�������ϣ���û����Һ��pH<7����˵������ͬ������MOH�ĵ���̶�______MCl��ˮ��̶ȡ�(�>������<������)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com