
���� ��1������c=$\frac{1000�Ѧ�}{M}$�������������Ϊ65%���ܶ�Ϊ1.4g/cm3��Ũ�����Ũ�ȣ�Ȼ�������Һϡ��ǰ�����ʵ����ʵ���������������3mol/L������100mL����Ҫ��Ũ����������
��2��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O����4NO+3O2+2H2O��4HNO3����ӵõ�4NH3+8O2=4HNO3+4H2O���ݴ˼������������������
��3������ת��Ϊ���ʵ���������NH3��HNO3��������������ʵ���������V=$\frac{m}{V}$����������Һ���ܶȣ����ݹ�ʽC=$\frac{n}{V}$������������ʵ���Ũ�ȣ�
��4�������������к���xmolNO��ymolNO2�������������n��NO����n��NO2��=9��1����������Ϊ3160mg������������������ʵ�����
����������һ���������塢��������������巴Ӧ������ʽΪ��NO+NO2+2NaOH=2NaNO2+H2O����Ӧ��ʣ������Ϊһ����������ʣ����һ������Ϊ�������������ݵ�ʧ�����غ��������һ�������õ���������������ݸ��������������ĺ���������Ҫ���������������
�����ݵ�ʧ�����غ��֪NO��NO2�е�ԭ�ӱ�Ϊ������0�۵�N �õ�������������NH3��-3�۵�N��Ϊ������0�۵�Nʧȥ�����������ݴ˽��
��� �⣺��1����������Ϊ65%���ܶ�Ϊ1.4g/cm3��Ũ�����Ũ��Ϊ��c=$\frac{1000��1.4��65%}{63}$mol/L=14.4mol/L��
��Һϡ��ǰ�����ʵ����ʵ������䣬��CŨ•VŨ=Cϡ•Vϡ
14.4mol/L•VŨ=3mol/L•0.1L
��ã�VŨ=0.0208L=20.8ml��
�ʴ�Ϊ��14.44 mol/L�� 20.8 ml��
��2��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O����4NO+3O2+2H2O��4HNO3����ӵõ�4NH3+8O2=4HNO3+4H2O�������������������Ϊ��$\frac{4��63}{4��63+4��18}$��100%=0.78��
�ʴ�Ϊ��78%��
��3��1.70��Һ�������ʵ���Ϊ��$\frac{1.7��1{0}^{6}}{17}$=0.1��106mol������ת����ϵ4NH3+8O2=4HNO3+4H2O����֪���ɵ���������ʵ���Ϊ0.1��106mol���������������ʵ���Ϊ��0.2��106mol�����ݷ���ʽ4NH3+8O2=4HNO3+4H2O����֪������Һ������=Һ��������+ˮ������+��������������=1.70��106+4.50��106+��32��0.2��106=12.6��106��g��
��������Һ�����V=$\frac{m}{��}$=$\frac{12.6��1{0}^{6}}{1.31��1{0}^{3}}$=9.62��103��L������C��HNO3��=$\frac{n}{V}$=$\frac{0.1��1{0}^{6}}{9.62��1{0}^{3}}$=10.40��mol/L����
�ʴ�Ϊ��10.40��
��4�����������֪��n��NO����n��NO2��=9��1����������Ϊ3160mg�������������к���xmolNO��ymolNO2��
��x��y=9��1��30x+46y=3.16�����x=0.09��y=0.01��
��һ���������塢��������������巴Ӧ������ʽΪ��NO+NO2+2NaOH=2NaNO2+H2O��
1 1
0.01mol 0.01mol
ʣ��NO���ʵ���Ϊ0.09mol-0.01mol=0.08mol��Ҫ����ȫ�����գ���ֻ��Ҫ����������0.04molNO����0.04mol�������������ݵ�ʧ�����غ�ã�0.04mol
����4-2��=n��O2����4�����n��O2��=0.02mol������������0.02mol��22.4L/mol=0.448L��
������������O2���������Ϊ0.25��������Ҫ�������������Ϊ��0.448L��0��25=1.792L��
��������Ҫ�����״���µĸ�������1.792L��
���ɢ�֪1m3���Ṥҵ��β���к�3160mg NOx������NO0.09mol������NO20.01mol������ð�����ԭ�������ð�����������NO��NO2ת��Ϊ����ֱ����������У�����Ҫ���������ʵ���Ϊ��n��NH3�������ݵ�ʧ�����غ��֪��0.09mol����2-0��+0.01mol����4-0��=n��NH3������0-��-3������
���n��NH3��=$\frac{0.22}{3}$��mol��
��������$\frac{0.22}{3}$mol��17g/mol=1.25g��
�𣺰���������Ϊ1.25g��
���� ���⿼�����йط���ʽ�ļ��㣬��ȷ����ʽ�и����ʵ����ʵ����Ĺ�ϵ�����ʵ������ǽ���ؼ���ע��ԭ�Ӹ�������ʧ�������غ���ɵ�Ӧ�ã���Ŀ�ѶȽϴ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��ѧʽ | ���볣����25�棩 |
| HCN | K=4.9��10-10 |
| CH3COOH | K=1.8��10-5 |
| H2CO3 | K1=4.3��10-7��K2=5.6��10-11 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2SO4��Һ | B�� | AgNO3��Һ | C�� | NaOH��Һ | D�� | KSCN��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
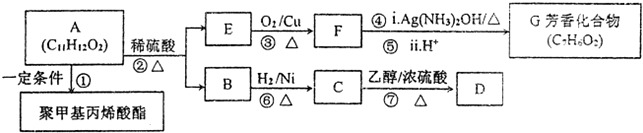
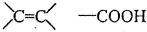 �������乬�������û�ѧ�Լ���������Ȼ�̼�������Ը��������Һ����̼��������Һ����ʯ���Լ�����
�������乬�������û�ѧ�Լ���������Ȼ�̼�������Ը��������Һ����̼��������Һ����ʯ���Լ����� ��
�� ��CH3��2CHCOOCH2CH3+H2O��
��CH3��2CHCOOCH2CH3+H2O��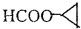 ��
��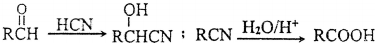
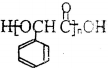 ��������д�м���Ľṹ��ʽ���ڡ������Ϸ����·�д��Ӧ�����������Լ���
��������д�м���Ľṹ��ʽ���ڡ������Ϸ����·�д��Ӧ�����������Լ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �٢� | C�� | �ڢ� | D�� | �ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
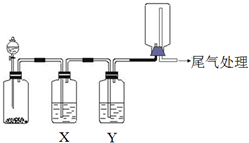 ������ͼװ�ÿ��Խ���ʵ�鲢�ܴﵽʵ��Ŀ���ǣ�������
������ͼװ�ÿ��Խ���ʵ�鲢�ܴﵽʵ��Ŀ���ǣ�������| ѡ�� | ʵ��Ŀ�� | X���Լ� | Y���Լ� |
| A | ��MnO2��Ũ������ȡ���ռ����������Cl2 | ����ʳ��ˮ | Ũ���� |
| B | ��Cu��ϡ������ȡ���ռ����������NO | ˮ | Ũ���� |
| C | ��֤��ʯ�뱥��ʳ��ˮ��Ӧ���ɵ���������ʲ��ռ� | CuSO4��Һ | KMnO4 ��Һ |
| D | CaCO3��ϡ������ȡ���ռ����������CO2 | ����NaHCO3��Һ | Ũ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
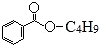 ������˵��������ǣ�������
������˵��������ǣ�������| A�� | X������ˮ | B�� | X�ķ���ʽΪC11H14O2 | ||
| C�� | ���Ϊ-C4H9��������3�� | D�� | X�ܷ����ӳɷ�Ӧ��ȡ����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ᡢ���Ư�� | B�� | ���ᡢ�ռС�մ� | ||
| C�� | �������ơ���ʯ�ҡ������� | D�� | ���ᡢ��ʯ�ҡ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
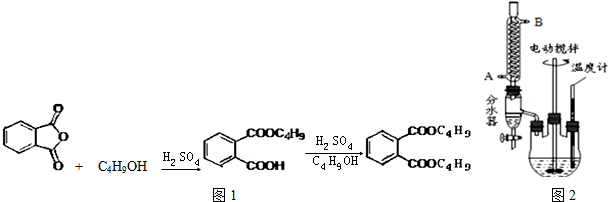
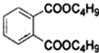 +2NaOH$\stackrel{��}{��}$
+2NaOH$\stackrel{��}{��}$ +2CH3CH2CH2CH2OH��
+2CH3CH2CH2CH2OH���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com