
Ńõ»ÆŠæĪŖ°×É«·ŪÄ©£¬æÉÓĆÓŚŹŖÕī”¢Ń¢µČʤ·ō²”µÄÖĪĮĘ”£“æ»Æ¹¤Ņµ¼¶Ńõ»ÆŠæ(ŗ¬ÓŠFe(¢ņ), Mn(¢ņ), Ni(¢ņ)µČŌÓÖŹ)µÄĮ÷³ĢČēĻĀ:
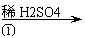 | 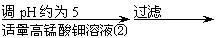 | 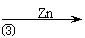 |
¹¤ŅµZnO ½ž³öŅŗ ĀĖŅŗ
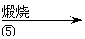 | |||||
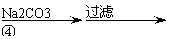 | |||||
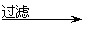 | |||||
ĀĖŅŗ ĀĖ±ż ZnO
ĢįŹ¾:ŌŚ±¾ŹµĮ³Ģõ¼žĻĀ£¬Ni(¢ņ)²»Äܱ»Ńõ»Æ£»øßĆĢĖį¼ŲµÄ»¹Ō²śĪļŹĒMnO2
»Ų“šĻĀĮŠĪŹĢā:
(1)·“Ó¦¢ŚÖŠ³żµōµÄŌÓÖŹĄė×ÓŹĒ £¬·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ £»
¼ÓøßĆĢĖį¼ŲČÜŅŗĒ°£¬ČōpH½ĻµĶ£¬¶Ō³żŌÓµÄÓ°ĻģŹĒ £»
(2)·“Ó¦¢ŪµÄ·“Ó¦ĄąŠĶĪŖ ”£¹żĀĖµĆµ½µÄĀĖŌüÖŠ£¬³żĮĖ¹żĮæµÄŠæĶā»¹ÓŠ £»
(3)·“Ó¦¢ÜŠĪ³ÉµÄ³ĮµķŅŖÓĆĖ®Ļ“£¬¼ģŃé³ĮµķŹĒ·ńĻ“µÓøɾ»µÄ·½·ØŹĒ
ӣ
(4)·“Ó¦¢ÜÖŠ²śĪļµÄ³É·ÖæÉÄÜŹĒZnCO3”¤xZn(OH)2 .Č”øɲŁŗóµÄĀĖ±ż11.2g£¬ģŃÉÕŗóæɵƵ½²śĘ·8.1 g. ŌņxµČÓŚ ”£
“š°ø£ŗ£Ø1£©Fe2£«ŗĶMn2£«£»
MnO4££«3Fe2£«£«7H2O£½MnO2”ż£«3Fe(OH)3”ż£«5H£«£»
3Mn2£«£«2MnO4££«2H2O£½5MnO2”ż£«4H£«£»
ĢśĄė×ÓŗĶĆĢĄė×Ó²»ÄÜÉś³É³Įµķ£¬“Ó¶ųĪŽ·Ø³żČ„ĢśŗĶĆĢŌÓÖŹ”£
£Ø2£©ÖĆ»»·“Ó¦£»Äų
£Ø3£©Č”×īŗóŅ»“ĪĻ“µÓŅŗÉŁĮæÓŚŹŌ¹ÜÖµ£¬µĪČė1”«2µĪĻ”ĻõĖį£¬ŌŁ“śČėĻõĖį±µČÜŅŗ£¬ČōĪŽ°×É«³ĮµķÉś³É£¬ŌņĖµĆ÷³ĮµķŅŃ¾Ļ“µÓøɾ»”£
£Ø4£©1



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ¹ŲÓŚ×ŌČ»½ēÖŠµŖŃ»·£ØČēÓŅĶ¼£©µÄĖµ·Ø²»Õż Č·µÄŹĒ£ŗ
Č·µÄŹĒ£ŗ
A”¢µ«ŌŖĖŲ¾ł±»Ńõ»Æ
B”¢¹¤ŅµŗĻ³É°±ŹōÓŚČĖ¹¤¹ĢµŖ
C”¢ŗ¬µŖĪŽ»śĪļŗĶŗ¬µŖÓŠ»śĪļæÉĻą»„×Ŗ»Æ
D”¢Ģ¼”¢Ēā”¢ŃõČżÖÖŌŖĖŲŅ²²ĪÓėĮĖµŖŃ»·
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÓŠ¹ŲČÜŅŗ×é³ÉµÄĆčŹöŗĻĄķµÄŹĒ(””””)
A£®ĪŽÉ«ČÜŅŗÖŠæÉÄÜ“óĮæ“ęŌŚAl3£«”¢NH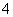 ”¢Cl£”¢S2£
”¢Cl£”¢S2£
B£®ĖįŠŌČÜŅŗÖŠæÉÄÜ“óĮæ“ęŌŚNa£«”¢ClO£”¢SO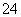 ”¢I£
”¢I£
C£®Čõ¼īŠŌČÜŅŗÖŠæÉÄÜ“óĮæ“ęŌŚNa£«”¢K£«”¢Cl£”¢HCO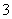
D£®ÖŠŠŌČÜŅŗÖŠæÉÄÜ“óĮæ“ęŌŚFe3£«”¢K£«”¢Cl£”¢SO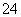
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĪļÖŹŠŌÖŹÓėÓ¦ÓƶŌÓ¦¹ŲĻµÕżČ·µÄŹĒ(””””)
A£®¾§Ģå¹čČŪµćøßÓ²¶Č“ó£¬æÉÓĆÓŚÖĘ×÷°ėµ¼Ģå²ÄĮĻ
B£®ĒāŃõ»ÆĀĮ¾ßÓŠČõ¼īŠŌ£¬æÉÓĆÓŚÖĘĪøĖįÖŠŗĶ¼Į
C£®ĘÆ°×·ŪŌŚæÕĘųÖŠ²»ĪČ¶Ø£¬æÉÓĆÓŚĘÆ°×Ö½ÕÅ
D£®Ńõ»ÆĢśÄÜÓėĖį·“Ó¦£¬æÉÓĆÓŚÖĘ×÷ŗģÉ«ĶæĮĻ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ņ»¶ØĮæµÄCuSŗĶCu2SµÄ»ģŗĻĪļĶ¶Čė×ćĮæµÄHNO3ÖŠ£¬ŹÕ¼Æµ½ĘųĢåVL£Ø±ź×¼×“æö£©£¬Ļņ·“Ó¦ŗóµÄČÜŅŗÖŠ£Ø“ęŌŚCu2+ŗĶSO42-£©¼ÓČė×ćĮæNaOH£¬²śÉśĄ¶É«³Įµķ£¬¹żĀĖ£¬Ļ“µÓ£¬×ĘÉÕ£¬µĆµ½CuO12.0g£¬ČōÉĻŹöĘųĢåĪŖNOŗĶNO2µÄ»ģŗĻĪļ£¬ĒŅĢå»ż±ČĪŖ1©s1£¬ŌņVæÉÄÜĪŖ
A.9.0L B.13.5L C.15.7L D.16.8L
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅųĶŗĻ½š¹ć·ŗÓĆÓŚŗ½æÕ¹¤Ņµ”£“ÓĒŠøī·ĻĮĻÖŠ»ŲŹÕŅų²¢ÖʱøĶ»Æ¹¤²śĘ·µÄ¹¤ŅÕČēĻĀ£ŗ

£Ø×¢£ŗAl(OH)3ŗĶCu(OH)2æŖŹ¼·Ö½āµÄĪĀ¶Č·Ö±šĪŖ450”ęŗĶ80”ę£©
£Ø1£©µē½ā¾«Į¶ŅųŹ±£¬Ņõ¼«·“Ó¦Ź½ĪŖ £»ĀĖŌüAÓėĻ”HNO3·“Ó¦£¬²śÉśµÄĘųĢåŌŚæÕĘųÖŠŃøĖŁ±äĪŖŗģ×ŲÉ«£¬øĆĘųĢå±äÉ«µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©¹ĢĢå»ģŗĻĪļBµÄ×é³ÉĪŖ £»ŌŚÉś³É¹ĢĢåBµÄ¹ż³ĢÖŠ£¬ŠčæŲÖĘNaOHµÄ¼ÓČėĮ棬ČōNaOH¹żĮ棬ŌņŅņ¹żĮæŅżĘšµÄ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
(3)Ķź³ÉģŃÉÕ¹ż³ĢÖŠŅ»øö·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ CuO + Al2O3 CuAlO2 + ”ü
CuAlO2 + ӟ
£Ø4£©ČōŅųĶŗĻ½šÖŠĶµÄÖŹĮæ·ÖŹżĪŖ63.5%£¬ĄķĀŪÉĻ5.0kg·ĻĮĻÖŠµÄĶæÉĶźČ«×Ŗ»ÆĪŖ mol CuAlO2£¬ÖĮÉŁŠčŅŖ1.0mol”¤L-1µÄAl2(SO4)3ČÜŅŗ L”£
(5)CuSO4ČÜŅŗŅ²æÉÓĆÓŚÖʱøµØ·Æ£¬Ę仳±¾²Ł×÷ŹĒ ”¢¹żĀĖ”¢Ļ“µÓŗĶøÉŌļ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ņ»ĻīæĘѧъ¾æ³É¹ū±ķĆ÷£¬ĶĆĢŃõ»ÆĪļ(CuMn2O4)ÄÜŌŚ³£ĪĀĻĀ“ß»ÆŃõ»ÆæÕĘųÖŠµÄŅ»Ńõ»ÆĢ¼ŗĶ¼×Č©(HCHO)”£
(1)ĻņŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄCu(NO3)2ŗĶMn(NO3)2ČÜŅŗÖŠ¼ÓČėNa2CO3ČÜŅŗ£¬ĖłµĆ³Įµķ¾øßĪĀ×ĘÉÕ£¬æÉÖʵĆCuMn2O4”£
¢ŁMn2£«»łĢ¬µÄµē×ÓÅŲ¼Ź½æɱķŹ¾ĪŖ ”£
¢ŚNO3£µÄæռ乹ŠĶ (ÓĆĪÄ×ÖĆčŹö)”£
(2)ŌŚĶĆĢŃõ»ÆĪļµÄ“ß»ÆĻĀ£¬CO±»Ńõ»Æ³ÉCO2£¬HCHO±»Ńõ»Æ³ÉCO2ŗĶH2O”£
¢Łøł¾ŻµČµē×ÓŌĄķ£¬CO·Ö×ӵĽį¹¹Ź½ĪŖ ”£
¢ŚH2O·Ö×ÓÖŠOŌ×Ó¹ģµĄµÄŌÓ»ÆĄąŠĶĪŖ ”£
¢Ū1molCO2ÖŠŗ¬ÓŠµÄ¦Ņ¼üŹżÄæĪŖ ”£
(3)ĻņCuSO4ČÜŅŗÖŠ¼ÓČė¹żĮæNaOHČÜŅŗæÉÉś³É[Cu(OH)4]2£”£²»æ¼ĀĒæռ乹ŠĶ£¬[Cu(OH)4]2£µÄ½į¹¹æÉÓĆŹ¾ŅāĶ¼±ķŹ¾ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌĖÓĆĻą¹Ų»ÆѧÖŖŹ¶½ųŠŠÅŠ¶Ļ£¬ĻĀĮŠ½įĀŪ“ķĪóµÄŹĒ(””””)
A£®Ä³ĪüČČ·“Ó¦ÄÜ×Ō·¢½ųŠŠ£¬Ņņ“ĖøĆ·“Ó¦ŹĒģŲŌö·“Ó¦
B£®NH4FĖ®ČÜŅŗÖŠŗ¬ÓŠHF£¬Ņņ“ĖNH4FČÜŅŗ²»ÄÜ“ę·ÅÓŚ²£Į§ŹŌ¼ĮĘæÖŠ
C£®æÉČ¼±łÖ÷ŅŖŹĒ¼×ĶéÓėĖ®ŌŚµĶĪĀøßŃ¹ĻĀŠĪ³ÉµÄĖ®ŗĻĪļ¾§Ģ壬Ņņ“ĖæÉ“ęŌŚÓŚŗ£µ×
D£®Ōö“ó·“Ó¦ĪļÅضČæɼÓæģ·“Ó¦ĖŁĀŹ£¬Ņņ“ĖÓĆÅØĮņĖįÓėĢś·“Ó¦ÄÜŌö“óÉś³ÉH2µÄĖŁĀŹ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÅŠ¶ĻŹŠĆęÉĻµÄ½šŹ×ŹĪŹĒ·ńŗ¬ÓŠĶ£¬æÉŅŌȔѳʷÓėijŹŌ¼Į½ųŠŠ·“Ó¦£¬øł¾ŻĻÖĻó¼“æÉÅŠ¶Ļ£¬øĆŹŌ¼ĮŹĒ£Ø £©
A£®ÅØŃĪĖį B£®ĻõĖį C£®ĶõĖ® D£®Ļ”ĮņĖį
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com