



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ʵ����� | Ԥ����������� |
| ����1��ȡ����������Һ����С�ձ��У��ý�ͷ�ι�ȡ1mol/L BaCl2��Һ��С�ձ��μ�ֱ�������� | �����ְ�ɫ���ǣ�����Һ�д���SO32-�� SO42-�� |
| ����2����С�ձ��е���Һ���ˡ�ϴ�ӣ���������ˮ�Ѹ�����ֽ�ϵĹ��������һС�ձ��У�����µĹ��� ����1�Σ���������Ʒ�� ����1�Σ���������Ʒ�� �ٵ������2mol/L���ᣬ�� �ٵ������2mol/L���ᣬ�� �� |
��Ʒ����ɫ���������ݣ���������Һ���д��� SO32-�� ��Ʒ����ɫ���������ݣ���������Һ���д��� SO32-�� ��Ʒ�첻��ɫ�����������ݣ���������Һ���в����� SO32- ��Ʒ�첻��ɫ�����������ݣ���������Һ���в����� SO32- |
| ����3�� ���Թ�ȡ������Һ ���Թ�ȡ������Һ �����е��������1mol/LBa��OH��2��Һ[�����1�Σ���������Ʒ����Һ���ٵ���2-3�Σ����������2mol/L����]���� �����е��������1mol/LBa��OH��2��Һ[�����1�Σ���������Ʒ����Һ���ٵ���2-3�Σ����������2mol/L����]���� �� |
�����ְ�ɫ��������Ʒ����Һ��ɫ���������ݣ���������Һ���д��� HSO3-�� �����ְ�ɫ��������Ʒ����Һ��ɫ���������ݣ���������Һ���д��� HSO3-�� �������ְ�ɫ��������Ʒ����Һ����ɫ����û�����ݣ���������Һ���в����� HSO3-�� �������ְ�ɫ��������Ʒ����Һ����ɫ����û�����ݣ���������Һ���в����� HSO3-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
2- 3 |
2- 4 |
- 3 |
2- 3 |
- 3 |
| ʵ����� | Ԥ����������� | ||||
| ����1��ȡ����������Һ����С�ձ��У��ý�ͷ�ι�ȡ1mol/L BaCl2��Һ��С�ձ��μ�ֱ�������� | �����ְ�ɫ���ǣ�����Һ�д��� SO
| ||||
| ����2����С�ձ��е���Һ���ˡ�ϴ�ӣ���������ˮ�Ѹ�����ֽ�ϵĹ��������һС�ձ��У�����µĹ��� |
|||||
| ����3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���¿α���������¿���������ѧ�Ծ���A�������������� ���ͣ�ʵ����
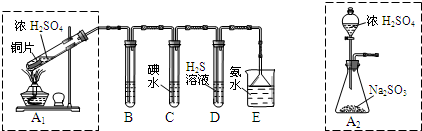
[2012���㶫������һģ]��14�֣�I��ij��ȤС��������ɫ������������̽��������Ⱦ��SO2�����ʣ��������ͼʵ��װ�á���ش�
��1��B��C��D�ֱ����ڼ���SO2��Ư���ԡ���ԭ�Ժ������ԣ���B����ʢ�Լ�Ϊ ��
C�з�Ӧ�����ӷ���ʽΪ ��
��2��Ϊ��ʵ����ɫ������Ŀ�꣬ijͬѧ�������ͼA2����ȡװ��������A1װ�ã���A1װ����ȣ�A2װ�õ��ŵ��� ��д���㣩��
II���ð�ˮ����β���е�SO2��������Һ���п��ܺ���OH-��SO32-��SO42-��HSO3-�������ӡ���ش�
��3����ˮ���չ���SO2�ķ�Ӧ�����ӷ���ʽΪ ��
��4����֪����������һ��������ˮ��SO2Ҳ������ˮ�������������Լ�Ϊ��С�ձ����Թܡ�����������ͷ�ιܡ�����װ�ú���ֽ��2 mol/L���ᡢ2 mol/LHNO3��1 mol/LBaCl2��Һ��l mol/LBa(OH)2��Һ��Ʒ����Һ������ˮ�������ʵ��֤��������Һ���д���SO32-��HSO3-������±���ʵ�������Ԥ������ͽ��ۣ�
| ʵ����� | Ԥ����������� |
| ����1��ȡ����������Һ����С�ձ��У��ý�ͷ�ι�ȡl mol/L BaCl2��Һ��С�ձ��μ�ֱ�������� | �����ְ�ɫ���ǣ�����Һ�д���SO32-�� SO42-�� |
| ����2����С�ձ��е���Һ���ˡ�ϴ�ӣ���������ˮ�Ѹ�����ֽ�ϵĹ��������һС�ձ��У�����µĹ��� �� | �� |
| ����3�� �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ɽ��ʡ����У������һ��������ѧ�Ծ��������棩 ���ͣ�ʵ����
����������һ�ִ�����Ⱦ��о���NO2��SO2��CO�ȴ�����Ⱦ����Ĵ�������Ҫ���壬ij��ѧʵ�鰮��С����̽��SO2�����ʣ�������·�����

��1��B��C��D�ֱ����ڼ���SO2�Ļ�ԭ�ԡ������Ժ�Ư���ԡ�����B��C�ֱ�Ϊ��ˮ�������ˮ��Һ����D����ʢ�Լ�Ϊ_________��B�з�Ӧ�����ӷ���ʽΪ��_________________��
��2��Ϊ��ʵ����ɫʵ���Ŀ�꣬ijͬѧ���������������ͼA2����ȡװ��������A1װ�ã���A1װ����ȣ�A2װ�õ��ŵ��ǣ�________________________________����дһ�㼴�ɣ���
��3��E���ð�ˮ����β���е�SO2��������Һ���п��ܺ���OH����SO32����SO42����HSO3���������ӡ���֪����������һ��������ˮ��SO2Ҳ������ˮ�������������Լ�Ϊ��С�ձ����Թܡ�����������ͷ�ιܡ�����װ�ú���ֽ��2mol/L���ᡢ1mol/L BaCl2��Һ��1mol/L Ba(OH)2��Һ��Ʒ����Һ������ˮ��
�����ʵ��֤��������Һ���д���SO32����HSO3��������±���ʵ�������Ԥ������ͽ��ۣ�
|
ʵ����� |
Ԥ����������� |
|
����1��ȡ����������Һ����С�ձ��У��ý�ͷ�ι�ȡ1mol/L BaCl2��Һ��С�ձ��μ�ֱ�������� |
�����ְ�ɫ���ǣ�����Һ�д���SO32���� SO42���� |
|
����2����С�ձ��е���Һ���ˡ�ϴ�ӣ���������ˮ�Ѹ�����ֽ�ϵĹ��������һС�ձ��У�����µĹ��� ___________________________________________________ |
_________________________ ______________________________________________ |
|
����3��_______ _______________________ ___________________________________________________ |
_________________________
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���¿α���������¿���������ѧ�Ծ���A�����������棩 ���ͣ�ʵ����
[2012���㶫������һģ]��14�֣�I��ij��ȤС��������ɫ������������̽��������Ⱦ��SO2�����ʣ��������ͼʵ��װ�á���ش�

��1��B��C��D�ֱ����ڼ���SO2��Ư���ԡ���ԭ�Ժ������ԣ���B����ʢ�Լ�Ϊ ��
C�з�Ӧ�����ӷ���ʽΪ ��
��2��Ϊ��ʵ����ɫ������Ŀ�꣬ijͬѧ�������ͼA2����ȡװ��������A1װ�ã���A1װ����ȣ�A2װ�õ��ŵ��� ��д���㣩��
II���ð�ˮ����β���е�SO2��������Һ���п��ܺ���OH-��SO32-��SO42-��HSO3-�������ӡ���ش�
��3����ˮ���չ���SO2�ķ�Ӧ�����ӷ���ʽΪ ��
��4����֪����������һ��������ˮ��SO2Ҳ������ˮ�������������Լ�Ϊ��С�ձ����Թܡ�����������ͷ�ιܡ�����װ�ú���ֽ��2 mol/L���ᡢ2 mol/LHNO3��1 mol/LBaCl2��Һ��l mol/LBa(OH)2��Һ��Ʒ����Һ������ˮ�������ʵ��֤��������Һ���д���SO32-��HSO3-������±���ʵ�������Ԥ������ͽ��ۣ�
|
ʵ����� |
Ԥ����������� |
|
����1��ȡ����������Һ����С�ձ��У��ý�ͷ�ι�ȡl mol/L BaCl2��Һ��С�ձ��μ�ֱ�������� |
�����ְ�ɫ���ǣ�����Һ�д���SO32-�� SO42-�� |
|
����2����С�ձ��е���Һ���ˡ�ϴ�ӣ���������ˮ�Ѹ�����ֽ�ϵĹ��������һС�ձ��У�����µĹ��� �� |
�� |
|
����3�� �� |
�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com