
 2Cu��+O2��+4H+����Һ�����ԣ�����ʯ����Һ���ɫ��
2Cu��+O2��+4H+����Һ�����ԣ�����ʯ����Һ���ɫ��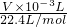 =
=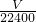 mol����Cu�����ԭ������ΪMr�����ݵ���ת���غ㣬��
mol����Cu�����ԭ������ΪMr�����ݵ���ת���غ㣬��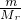 ��2=
��2=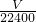 mol��4�����Mr=
mol��4�����Mr=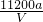 ��
��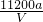 ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
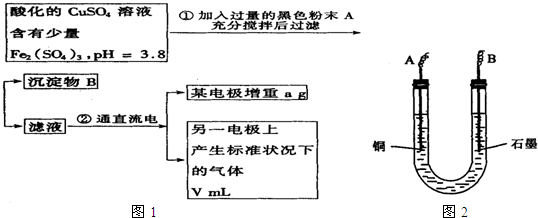
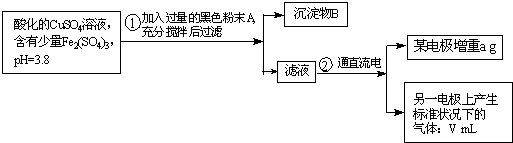
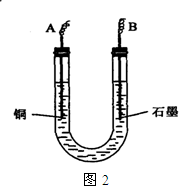
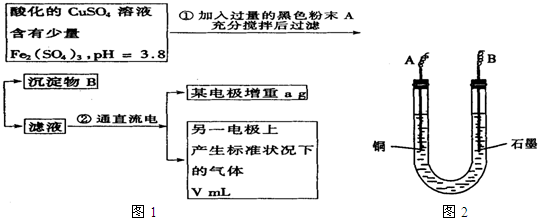
 ��֪��pHΪ4��5����Һ�У�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⣮ijѧ�����õ��CuSO4��Һ�ķ����ⶨͭ�����ԭ����������ͬѧ��pH=3.8�ữ�ġ�����Fe2��SO4��3���ʵ�CuSO4��Һ�м�������ĺ�ɫ��ĩX����ֽ������Һ����ͼ��ʾװ�õ�⣬����ij�缫����a g����һ�缫�ϲ�����״���µ�����VmL������˵����ȷ���ǣ�������
��֪��pHΪ4��5����Һ�У�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⣮ijѧ�����õ��CuSO4��Һ�ķ����ⶨͭ�����ԭ����������ͬѧ��pH=3.8�ữ�ġ�����Fe2��SO4��3���ʵ�CuSO4��Һ�м�������ĺ�ɫ��ĩX����ֽ������Һ����ͼ��ʾװ�õ�⣬����ij�缫����a g����һ�缫�ϲ�����״���µ�����VmL������˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪��pHΪ4��5����Һ�У�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⣮ijͬѧ���õ������ͭ��Һ�ķ����ⶨͭ�����ԭ����������ͬѧ��pH=3.8�ĺ������������ʵ�����ͭ��Һ�м�������ĺ�ɫ��ĩX����ֽ������Һ����ͼ��ʾװ�õ�⣬����ij�缫����a g����һ�缫�ϲ�����״���µ�����V mL������˵����ȷ���ǣ�������
��֪��pHΪ4��5����Һ�У�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⣮ijͬѧ���õ������ͭ��Һ�ķ����ⶨͭ�����ԭ����������ͬѧ��pH=3.8�ĺ������������ʵ�����ͭ��Һ�м�������ĺ�ɫ��ĩX����ֽ������Һ����ͼ��ʾװ�õ�⣬����ij�缫����a g����һ�缫�ϲ�����״���µ�����V mL������˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��pHΪ4��5����Һ�У�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⡣ijѧ�����õ��CuSO4��Һ�ķ����ⶨͭ�����ԭ����������ͬѧ��pH=3.8�ữ�ġ�����Fe2��SO4��3���ʵ�CuSO4��Һ�м�������ĺ�ɫ��ĩX����ֽ������Һ��ͼ��ʾװ�õ�⣬����ij�缫����a g����һ�缫�ϲ�����״���µ�����VmL������˵����ȷ���ǣ� ��
A����ɫ��ĩX������
B��ͭ�缫���ӵ�Դ����
C��ʯī�缫�Ϸ����ķ�Ӧ��4OH-��4e-=O2����2H2O
D��ͭ�����ԭ�������ļ���ʽ��![]()
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com