
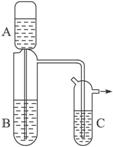
��������ҩƷ�ɹ�ѡ��Ũ���ᡢŨ���ᡢϡ��ˮ���廯����Һ����ˮ�Ҵ�������������Һ���Ȼ�����Һ��
��1��������ҩƷ��ѡ���Լ�����װ�ÿ���ȡ�������ǣ�д��ѧʽ����____________________��
��2����ȡʱ������ʹ����A�е��Լ�����ȫ����ëϸ���е��¶������ã���������Ӧװ����Լ��ǣ�A��_____________��B��_____________��
��3����ȡʱ����Ҫ��һ��ʼ�ͻ�þ����ȶ�����������������Ӧװ����Լ��ǣ�A��_____________��B��_____________��
��4����������ëϸ�ܵ�������__________________________��װ��C��Ӧװ����Լ�Ϊ_____________����������_______________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?�Ϻ���ʵ������ȡ�����������װ������ͼ��ʾ�������������������գ�
��2011?�Ϻ���ʵ������ȡ�����������װ������ͼ��ʾ�������������������գ�| ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
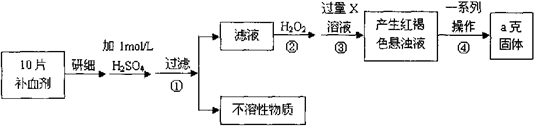
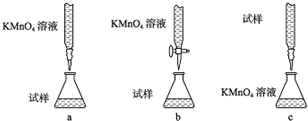
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ҩƷ�ɹ�ѡ��Ũ���ᡢŨ���ᡢϡ��ˮ���廯����Һ����ˮ�Ҵ�������������Һ���Ȼ�����Һ��

(1)������ҩƷ��ѡ���Լ�����װ�ÿ���ȡ��������(д��ѧʽ)��______________��
(2)��ȡʱ������ʹ����A�е��Լ�����ȫ����ëϸ���е��¶������ã���������Ӧװ����Լ��ǣ�A��______________��B��______________��
(3)��ȡʱ����Ҫ��һ��ʼ�ͻ�þ����ȶ�����������������Ӧװ����Լ��ǣ�A��______________��B��______________��
(4)��������ëϸ�ܵ�������_____________________________________��װ��C��Ӧװ����Լ�Ϊ______________����������__________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com